38 mo diagram for ch4
Molecular Orbital Diagram for CH4. Rename to desired sub-topic. You can delete the header for this section and place your own related to the topic. Remember to hyperlink your module to other modules via the link button on the editor toolbar. References. Housecroft, C; Sharpe, A. (2008). Bonding in Polyatomic Molecules. The overall molecular orbital energy level diagram for σ-bonding in octahedral complexes can be shown as: Figure 10. The formation of σ-molecular orbitals (bonding, antibonding and non-bonding) in octahedral complexes of transition metals. Buy the complete book with TOC navigation,
MO diagram-As we can see in this diagram, the energy level of 3 LGOs are higher than the 2s orbital and below the 2 p orbital dued to the electronegativy of both Boron and Hydrogen. Hydrogen has higher electronegativity than boron, therefore hydrogen would have lower energy level in the MO diagram.
Mo diagram for ch4
UCI Chem 131A Quantum Principles (Winter 2014)Lec 27. Quantum Principles -- CH4 Molecular Orbitals and Delocalized Bonding --View the complete course: http:... Build MO diagram. We expect six MOs, with the O 2pytotally nonbonding. Water H He Li Be B C N O F Ne B C N O F Ne Na Mg Al Si P S Cl Ar Al Si P S Cl Ar 1s 2s 2p 3s 3p -13.6 eV -15.8 eV -32.4 eV Based on the large ΔE, we expect O 2s to be almost nonbonding. Water With the orbital shapes, symmetries, and energies in hand we can make the MO ... Molecular Orbital of Methane, CH 4 1. The Lewis structure shows us that the carbon atom makes 4 sigma bonds to hydrogen and has no non-bonding electron pairs. 2. The central carbon atom combines its 2s, 2p x, 2p y, and 2p z valence orbitals to make four, 2sp3 hybrid orbitals. 3. Each one of these combines with a 1s atomic orbital from a ...
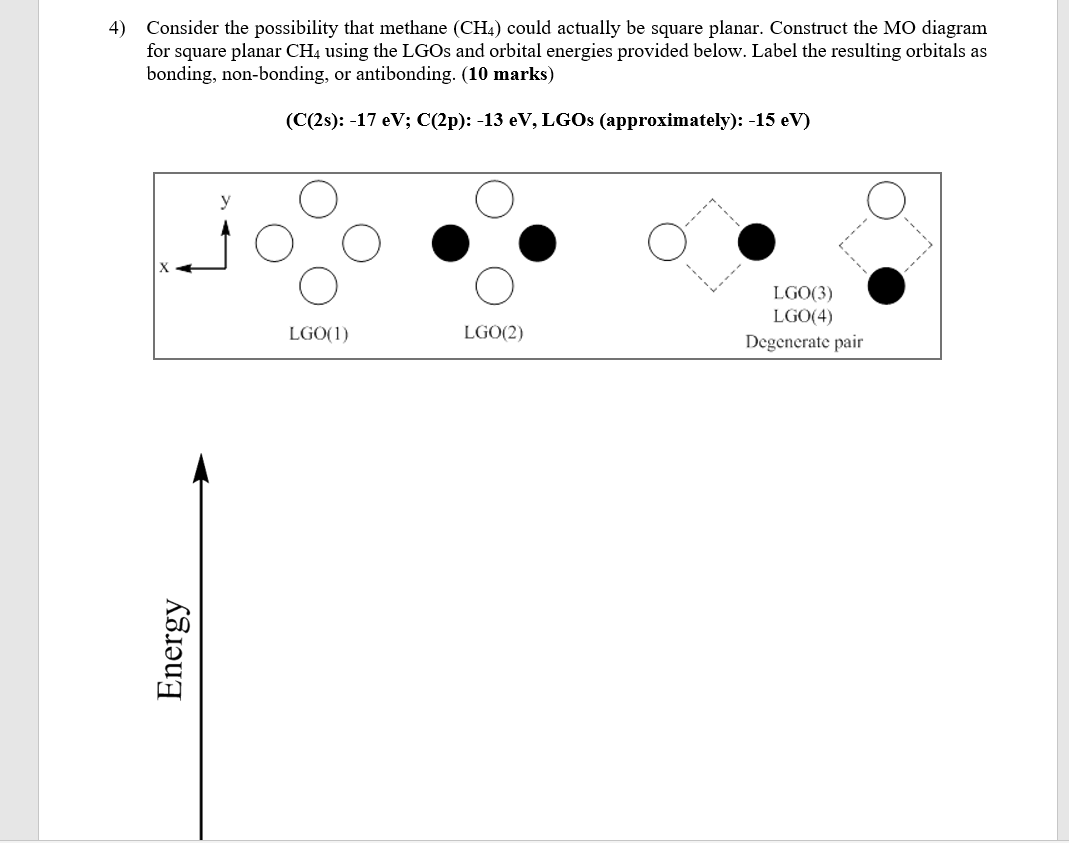
Mo diagram for ch4. a complex MO diagram: B2H6 MO diagrams combine two fragments. Symmetry fragments . these are MOs from C2H4 which belongs to the D2h point group. Answer to construct a molecular orbital diagram of C2H4 with molecular obital digrams of CH2 on both sides of C2H4 I think I have. Loop Diagram. 3dxy . Construct the molecular orbital diagram for ... One question I will ask them on the exam is to generate the MO diagram for AlH3, to really see if the understood the MO diagram for BH3. I know "AlH3" is really a polymer, but if they recognize that B and Al are in the same group, they could sketch a similar MO diagram. I then had them generate the LGOs for CH4 and the MO diagram. Construct an MO diagram for a square planar CH4. Comparing your diagram to the diagram of tetrahedral CH4 derived in class, explain why CH4 exists in the tetrahedral geometry and not the square planar geometry. Question: 4. Consider a theoretical molecule, square planar CHs. Below are the four LCAOs of the four H 1s atomic orbitals. Construct ... CH4 P. CH3OH. Which of the following compounds exhibits hydrogen bonding? CH3CH2OH CH3F (CH3)3N CH3OCH3. CH3CH2OH. Use the following MO diagram for Be2, Be2+, and Be2-. Based on this diagram, Be2+ and Be2- are both more stable than Be2. The paramagnetism of O2 is explained by resonance. coordinate covalent bonding. molecular orbital theory ...
Produce the molecular orbital diagram of the hypothetical square-planar CH4 molecule. Then produce the MO diagram for the tetrahedral CH_4 molecule. Make sure you organize MO energies in the tetrahedral version so that it has four C-H bonds. Why exactly is the planar CH_4 molecule unstable relative to the tetrahedral arrangement? Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the ... Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H Construction of MO diagrams for Transition Metal Complexes bonding only scenario. Example: Constructing a MO for Hexammine 2+Ruthenium, [[(Ru(NH 3) 6] NH 3 NH 3 2+ H 3N Ru NH 3 point group = O h H 3N NH 3 h 48 Ru bonding AOs O h E 8C 3 6C 2 6C 4 3C 2 i6S 4 8S 6 3 h 6 d /h 60 02 20004 2 = A 1g: 5s T
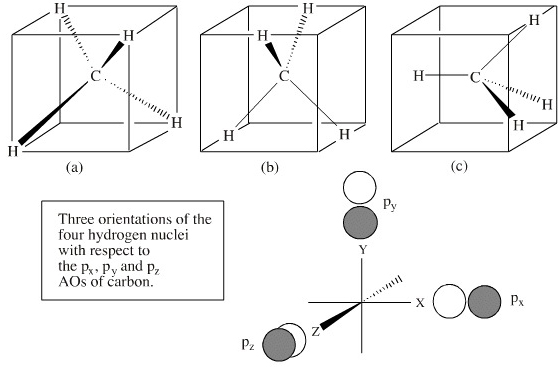
Question: 3. Molecular orbital theory. Methane (CH4) has tetrahedral geometry and Td point group symmetry. Derive a molecular orbital diagram for methane by performing the following steps: a. Determine the reducible representation (Γ) describing the symmetry of the four H 1s orbitals that are involved in σ bonding with the valence atomic ... C2H4 Molecular Orbital (MO) Diagram. The molecular orbital theory is a concept of quantum mechanics where atomic linearly combines to form molecular orbitals and we describe the wave nature of atomic particles. Here, bond strength depends on the overlapping degree which in turn depends on the spatial proximity of the combining atoms. Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals. 1. Begin with the Lewis Molecular Orbital of Methane, CH4. 1. The Lewis. Molecular Orbital theory (MO) is the most important quantum mechanical theory This particular diagram shows the orbitals for both the hydrogen atom and the. In order to understand the hybridization of CH 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. The type of hybridization involved with CH4 is sp 3. We will discuss in detail how this hybridization occurs below. Name of the Molecule. Methane. Molecular Formula. CH 4.
Molecular Orbital Diagram for Methane. Sigma and pi covalent bond models have proven to be valuable tools for describing the structure and reactivity of simple molecules, such as methane and ethene. However, such models do not accurately represent the electron distribution within the molecules. In the case of methane, this model implies four ...
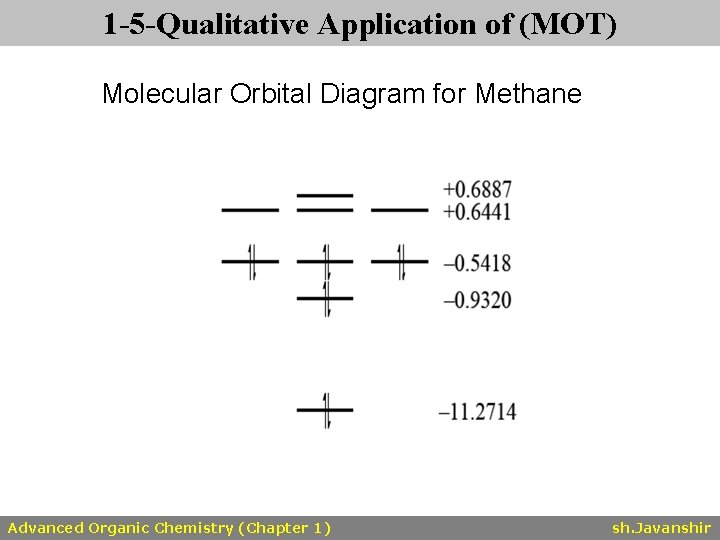
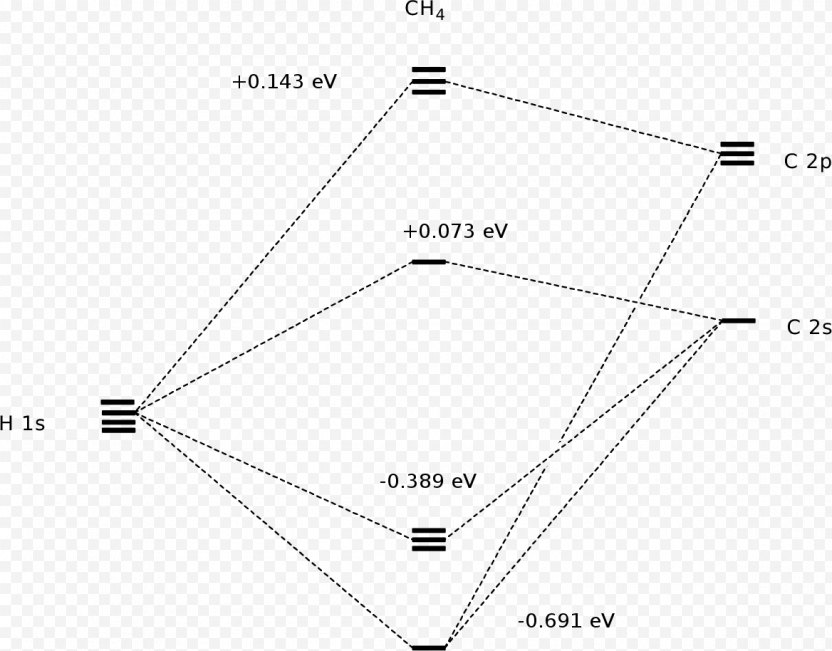
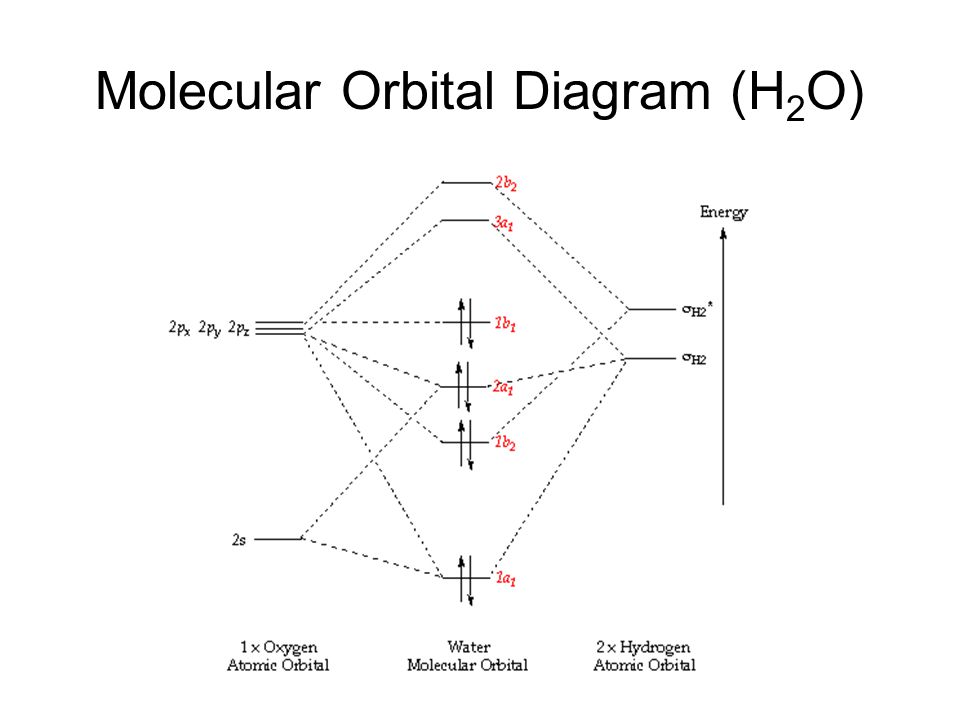
A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below. (McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p. 388) Methane has eight valence electrons, so according to the aufbau and Pauli exclusion principles the two
all the possible molecular orbitals in a structure, two are so special they get their own names. One is called the highest occupied molecular orbital (HOMO), because it is the highest energy orbital holding electrons. The other is called the lowest unoccupied molecular orbital (LUMO), because it is the lowest energy orbital without any electrons.
June 22, 2020 - Do you notice something missing, broken, or out of whack? Maybe you just need a little extra help using the Brand. Either way we would love to hear from you · Page content is the responsibility of Prof. Kevin P. Gable kevin.gable@oregonstate.edu 153 Gilbert Hall Oregon State University Corvallis ...
A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals.

Is S P Mixing Referring To Hybridization Or Is It The Mixing Of One Atoms S Orbital With The Other S P Orbital Chemistry Stack Exchange
#3. Draw the MO diagram for `O_2^+` This is a bit of a curveball, but a perfectly valid problem. Recall that a cation indicates a loss of `1` electron. `O_2^+` is just the ionized form of `O_2`; that is, it's `O_2` with `1` missing electron. The MO diagram will be the same as the MO diagram of `O_2`, except with `1` less electron.
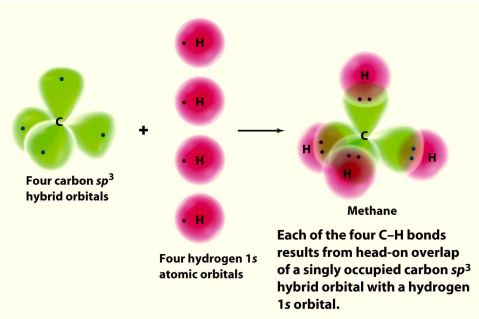
Methane is a pentatomic, tetrahedral molecule. It is formed by combination of one carbon atom with 4 hydrogen atoms. In the molecule of methane, the carbon a...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form ...
The energy levels in a hydrogen molecule can be represented in a diagram - showing how the two 1s atomic orbitals combine to form two molecular orbitals, one bonding (s) and one antibonding (s *). This is shown below - by clicking upon either the s or s * molecular orbital in the diagram - it will show graphically in a window to the right: 3.
Homepage of the John McGrady's Computational Inorganic Chemistry Group, Inorganic Chemistry Laboratory, South Parks Road, University of Oxford. The group's research interests focus on the electronic structure of inorganic systems in the broadest sense. We apply modern computational methods ...
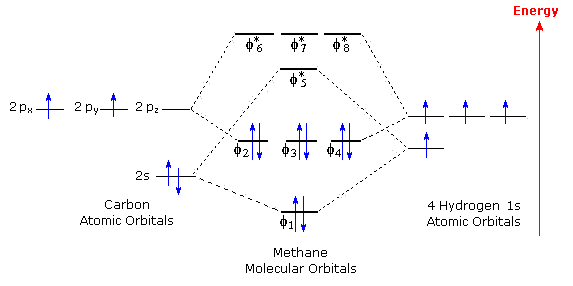
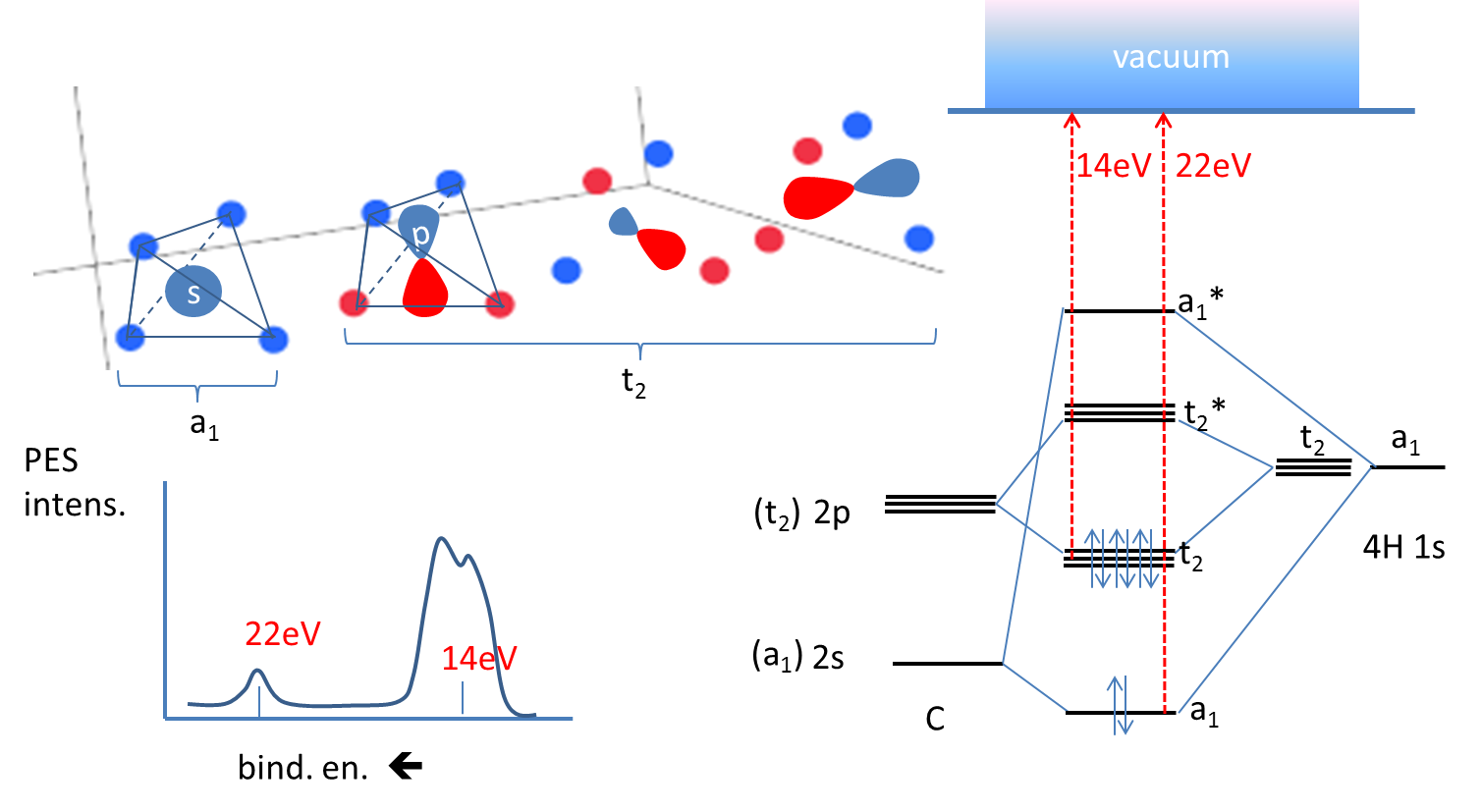
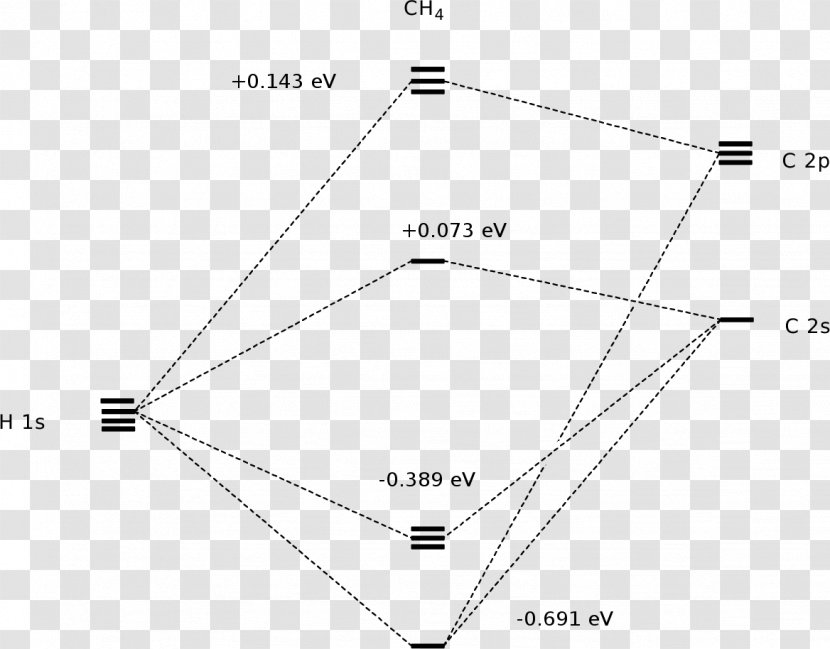
Introduction: Methane, CH 4. Using the carbon and hydrogen atomic orbitals, methane, CH 4, is constructed by overlapping the carbon's one 2s and three 2p AOs with the four hydrogen 1s AOs.. Methane's MOs have a topology similar to the AOs of carbon, but the structure can be very difficult to visualise, so the methane MO construction diagrams A, B and C (below) are shown with the AOs and MOs ...
CH4 in square planar (D4h) CH4 in tetrahedral shape (Td) The correlation diagram between the bonding MOs of square planar and tetrahedral CH4 is as follows: - During the D4h to Td distortion, the two degenerate energy levels in D4h are destabilized due to the overlap between the p orbital on ...
Solved Propose A Complete Molecular Orbital Diagram With All Of The Sigma And Pi Interactions For Ptcl 4 2 The Electron Configuration For The Sq Course Hero
August 15, 2020 - Most general chemistry textbooks invoke sp3 hybridization to explain the bonding in the tetrahedral methane (CH4) molecule. The idea (valence bond theory, VBT) is that good overlap between the atomic orbitals centered on carbon and hydrogen leads to strong bonds.
I have understood the formation of $\ce{CH4}$ by valance bond theory, but I'm having trouble understanding it through molecular orbital theory. The energy level diagram of molecular orbitals of $\ce{CH4}$ is not clear to me.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in . A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding. Example #8: EX4 type molecules in Td ...
Read more at: https://pubs.acs.org/doi/10.1021/acsomega.0c05499. ... An informational presentation by Dr. Adrian Schwan for prospective students interested in pursuing a degree in chemistry at the University of Guelph.
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we’ll see that symmetry will help us treat larger molecules in
Ch4 Mo Diagram you looking for is available for all of you right here. we have 12 examples on Ch4 Mo Diagram including images, pictures, models, photos, and more. On this website, we also have variation of photographs available. Such as png, jpg, animated gifs, pic art, logo, black and white, ...
This section illustrates pictorially molecular orbitals for several organic and inorganic molecules. If possible - the energy level diagram is included and clicking upon the relelvant level will generate the accompanying molecular orbital in the right-hand frame. Please choose from:
Molecular Orbital Theory. Chemists have been fascinated with square planar methane (CH4) since 1970. Use group theory and SALC (symmetry adapted linear combination) to generate the MO diagram. (a) Begin by determining the point group of the molecule and identifying the xyz axes.
Solved Generate The Mo Diagram For Ch4 As Square Planar And Ch4 As Tetrahedral Why Does Ch4 Not Exist As A Swaure Planar But It Does As A Tetrahed Course Hero
Read more at: https://pubs.acs.org/doi/10.1021/acsomega.0c05499. ... An informational presentation by Dr. Adrian Schwan for prospective students interested in pursuing a degree in chemistry at the University of Guelph.
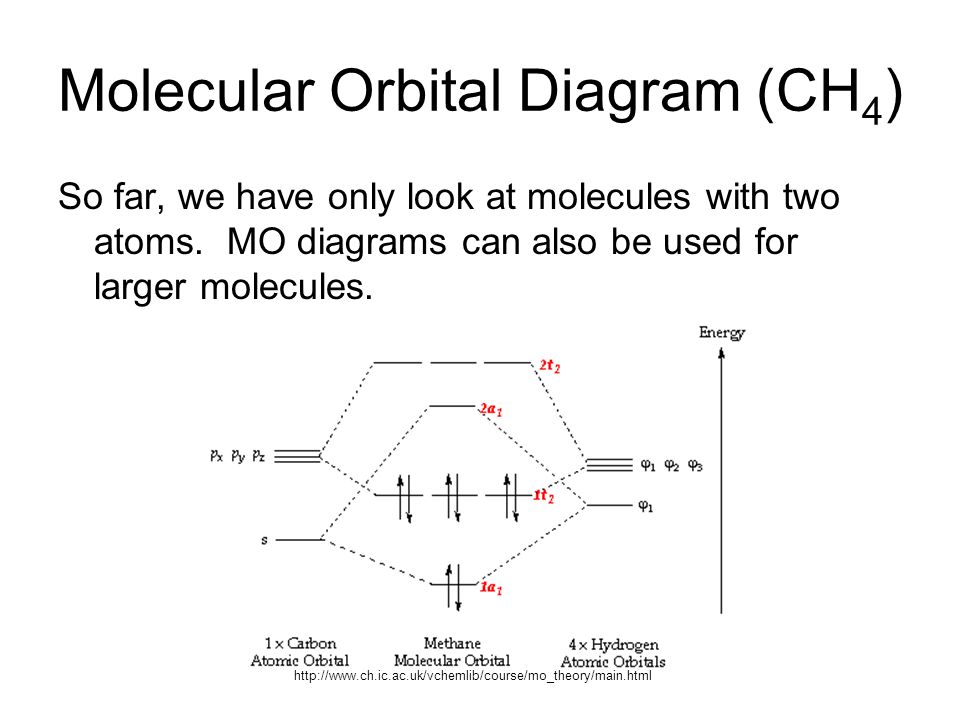
Drawing the Molecular Orbital Diagram. Figure 1.9. 2: Structure of methane molecule. Consider CH 4 as a specific example of a molecule within the Td symmetry group. Before drawing the MO diagram, the valence atomic orbitals on the central atom and their symmetries must be noted.
I obtained a BS in Chemistry from Youngstown State University in 1998. I completed a Ph.D. from the Ohio State University in 2002. My research advisor was Professor Patrick Woodward and my research at that time focused experimental solid state chemistry and on the development of a software ...
Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
Molecular Orbital (MO) Theory (continued 1) • Filling of MOs with electrons is governed by the same rules as for atomic orbitals • Aufbau principle - Fill MOs beginning with the lowest energy unoccupied molecular orbital • Pauli exclusion principle - No more than two electrons can be accommodated in a MO, and their spins must be paired
The molecular orbital diagram of CH 4 is shown below.. The hybridization of the methane molecule is sp 3.One 2s orbital and three 2p orbitals hybridize to form sp 3.The methane molecule forms molecular orbitals via linear combinations of the unhybridized carbon's 2s and 2p orbital with hydrogen's 1s orbital.
Molecular Orbital of Methane, CH 4 1. The Lewis structure shows us that the carbon atom makes 4 sigma bonds to hydrogen and has no non-bonding electron pairs. 2. The central carbon atom combines its 2s, 2p x, 2p y, and 2p z valence orbitals to make four, 2sp3 hybrid orbitals. 3. Each one of these combines with a 1s atomic orbital from a ...
Build MO diagram. We expect six MOs, with the O 2pytotally nonbonding. Water H He Li Be B C N O F Ne B C N O F Ne Na Mg Al Si P S Cl Ar Al Si P S Cl Ar 1s 2s 2p 3s 3p -13.6 eV -15.8 eV -32.4 eV Based on the large ΔE, we expect O 2s to be almost nonbonding. Water With the orbital shapes, symmetries, and energies in hand we can make the MO ...
UCI Chem 131A Quantum Principles (Winter 2014)Lec 27. Quantum Principles -- CH4 Molecular Orbitals and Delocalized Bonding --View the complete course: http:...









Comments
Post a Comment