42 reaction coordinate diagram rate determining step
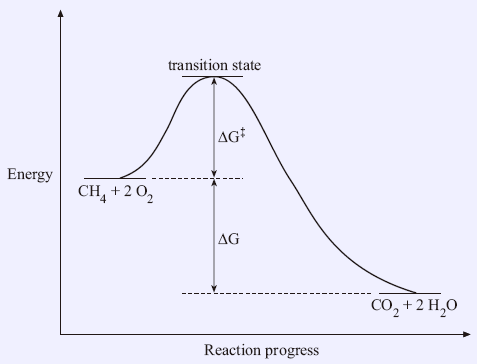
Given a reaction coordinate (energy diagram), the rate determining step can be determined by taking the largest energy difference between any starting material or intermediate on the diagram and any transition state that comes after it. That transition state will then be the rate-determining step of a given reaction. Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction . Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
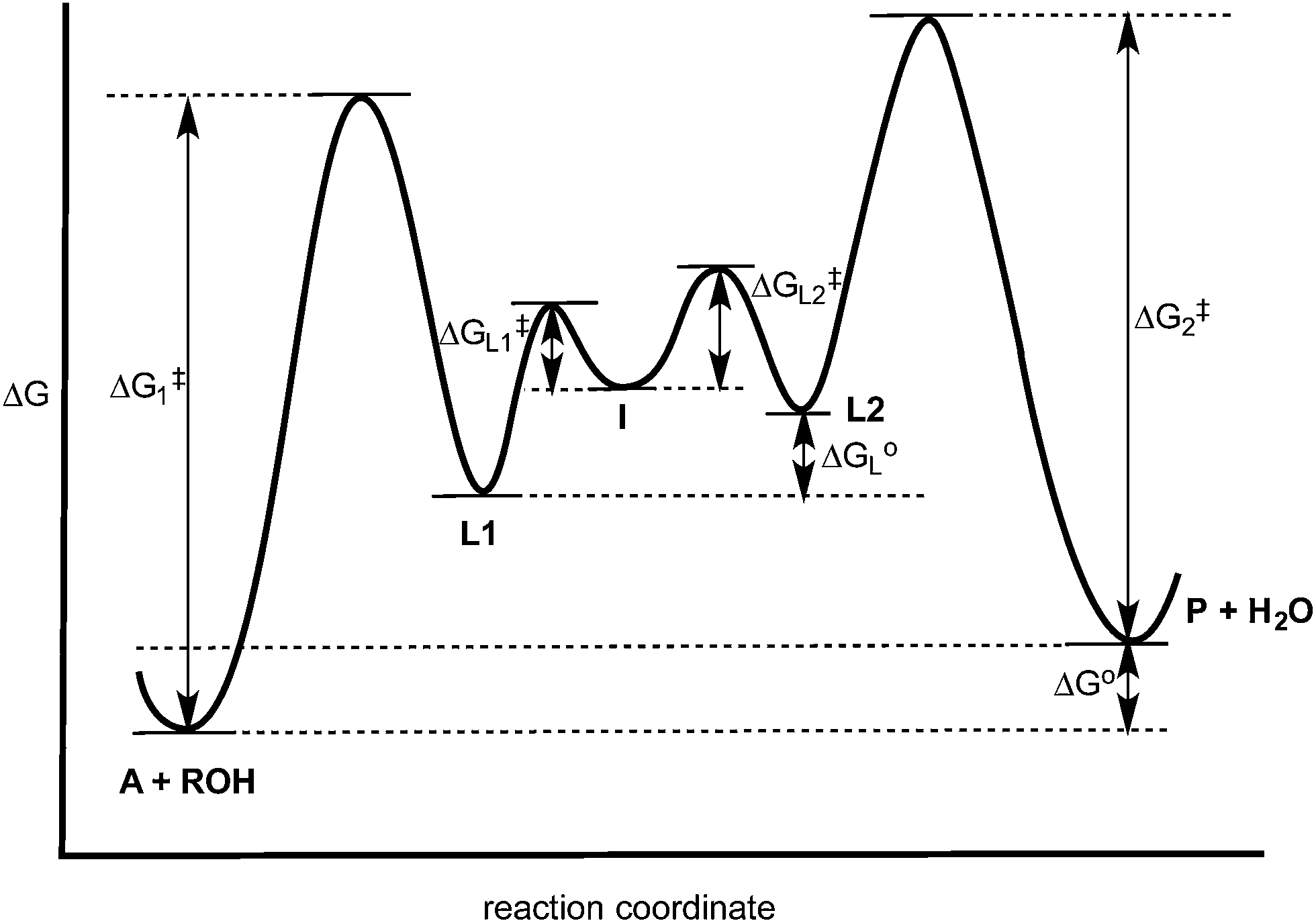
In a multistep reaction, the rate-determining step does not necessarily correspond to the highest Gibbs energy on the reaction coordinate diagram. [5] [3] If there is a reaction intermediate whose energy is lower than the initial reactants, then the activation energy needed to pass through any subsequent transition state depends on the Gibbs energy of that state relative to the lower-energy intermediate.
Reaction coordinate diagram rate determining step
12 Sept 2021 — In a reaction coordinate diagram, the vertical axis represents the ... of activation - is referred to as the rate-determining step (rds). 13 Aug 2016 · 1 answerThe rate determining step in a reaction mechanism is the slowest step. It is characterized by its high activation energy. Consider the energy ... The Arrhenius theory and reaction coordinates relate to a particular perspective on chemical kinetics that imagines the path taken by the reactants as they are converted to the products. This change is not instantaneous and is typically slowed by the fact that in order to convert from reactants to products, the system must first pass through a higher energy state known as the transition state. The size of the energy barrier controls the temperature dependence of the rate constant. At any given temperature there is a distribution of energies for the molecules in the system. Some small fraction have enough energy to get over this barrier. The reaction rate then depends on the number of molecules that have sufficient energy. To speed up the rate, one can either raise the temperature or one can or lower the barrier. As a result, essentially all chemical reaction increase in rate as the temperature is increased. This is the idea that is the basis for the Arrhenius Law. However, this has...
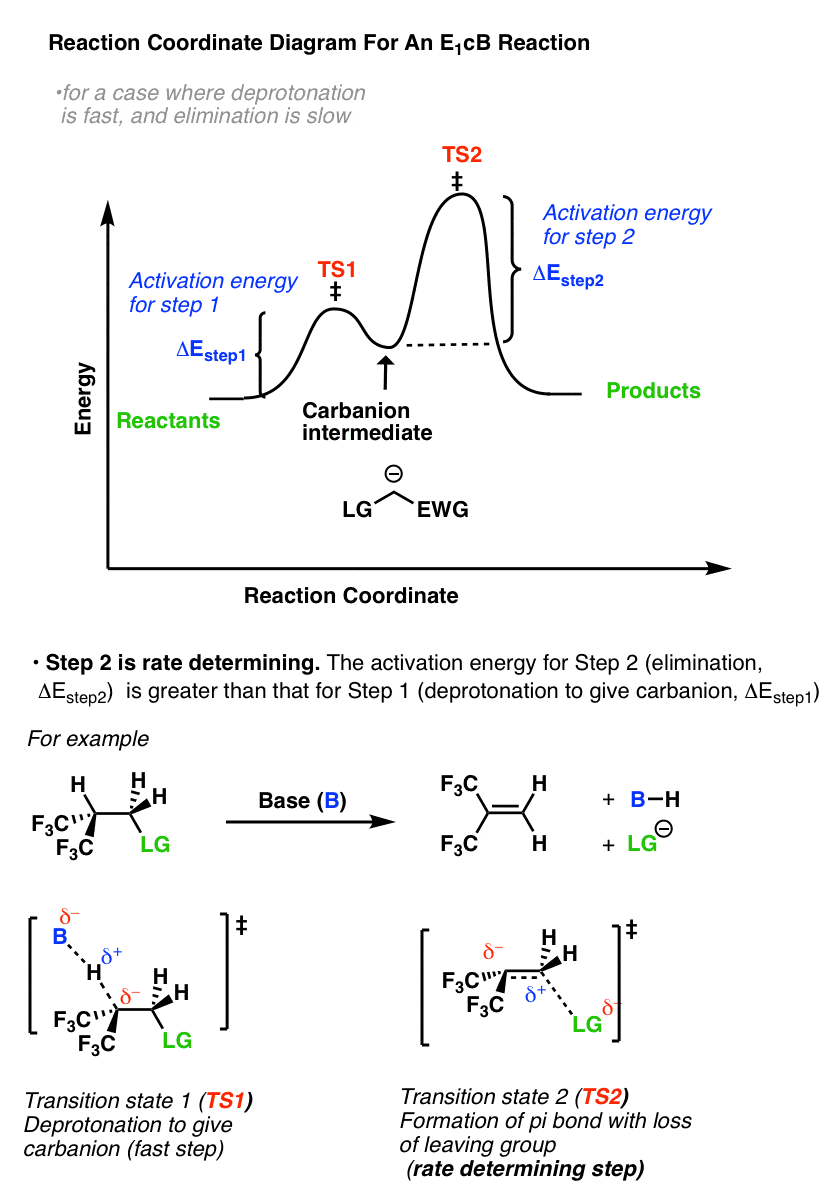
Reaction coordinate diagram rate determining step. Sketch out an activation energy diagram for a multistep mechanism involving a rate-determining ... Diagram. The reaction coordinate diagram is represented below: Things to note. ΔH represents the difference between enthalpy of reactants and products. Ea1 and Ea2 represent the activation energy for step 1 and step 2 in the reaction. The step with the highest activation energy is the slowest step reaction. The step with the lowest activation energy is the fastest step in the reaction. **|<-[Beginning](https://www.reddit.com/r/HFY/comments/4atv66/30000_changing_the_rules/) &nbsp; &nbsp; <<-[First Part](https://www.reddit.com/r/HFY/comments/645vow/rules_2_breaking_the_rules_part_15/) &nbsp; &nbsp; <-[Previous](https://www.reddit.com/r/HFY/comments/645wc6/rules_2_breaking_the_rules_part_25/) &nbsp; &nbsp; [Wiki](https://www.reddit.com/r/HFY/wiki/authors/aluminiumcomet) &nbsp; &nbsp; [Next](https://www.reddit.com/r/HFY/comments/64c680/r... When we look at a reaction coordinate diagram for a proposed mechanism, the first step is to identify the rate-determining (slowest) step. We identify the RDS as the step with the highest "peak," i.e. the transition state with the highest energy. Why doesn't the rate of a given step instead depend on its activation energy (Ea)? It would seem to stand to reason that, if we view each elementary step as an individual reaction, the slowest step would be that which must overcome the largest "jump" ...
Hope this is helpful to someone! Here's a debrief on my failed practical exam -I did not pass the flight portion and have 60 days to retest those maneuvers I did not pass and those which we were not able to get to. **Bottom line**: This was on me and I agree that I unsatisfactorily completed the forward slip to landing and my first landing was unsatisfactory. XXX is definitely a tough examiner and definitely not someone enjoyable to go through an oral with or a practical flight portion wit... Rate determining step (rds; rate limiting step): The mechanism step with the greatest activation energy (i.e., the slowest step) and therefore the step that ... Hello, I've gotten myself tangled in a mental knot here. In a reaction coordinate diagram, is the RDS the one with the greatest delta-G, or with the highest overall G value? Hey everyone, I’m Bill and I’m currently in my 2nd year of a BBiomed single degree here at Monash. A few months ago, I’ve put up an expression of interest for a biomed + chem study skills post à la allevana and luneax, but man, I’ve been swamped with uni work for the past 9 weeks (thank you very much Monash for that 3/4 sem break /s). I’ll write a quick paragraph on every single subject I did, but mainly focusing on pitfalls to beware of because these are probably the most valuable for future st...
A typical reaction coordinate diagram for a mechanism with a single step is ... coordinate diagrams is how to determine what the rate determining step is. The Arrhenius theory and reaction coordinates relate to a particular perspective on chemical kinetics that imagines the path taken by the reactants as they are converted to the products. This change is not instantaneous and is typically slowed by the fact that in order to convert from reactants to products, the system must first pass through a higher energy state known as the transition state. The size of the energy barrier controls the temperature dependence of the rate constant. At any given temperature there is a distribution of energies for the molecules in the system. Some small fraction have enough energy to get over this barrier. The reaction rate then depends on the number of molecules that have sufficient energy. To speed up the rate, one can either raise the temperature or one can or lower the barrier. As a result, essentially all chemical reaction increase in rate as the temperature is increased. This is the idea that is the basis for the Arrhenius Law. However, this has... 13 Aug 2016 · 1 answerThe rate determining step in a reaction mechanism is the slowest step. It is characterized by its high activation energy. Consider the energy ... 12 Sept 2021 — In a reaction coordinate diagram, the vertical axis represents the ... of activation - is referred to as the rate-determining step (rds).

E1 Energy Diagram Loss Of Leaving Group Is The Rate Determining Step Chemistry Lessons Organic Chemistry Chemistry
Solved A Reaction Coordinate Diagram Is Shown For The Reaction Of A To Form E Free Energy Reaction Coordinate Identify The Transition State S Wh Course Hero

Energy Diagrams A Review Energy Diagrams Are A Plot Of The Reaction Steps Or Reaction Coordinate X Axis Versus The Energy Kcal Or Kj Ppt Download

1 Describe The Reaction That Occurs At Modest Temperatures Between The Starting Material And Brainly Com
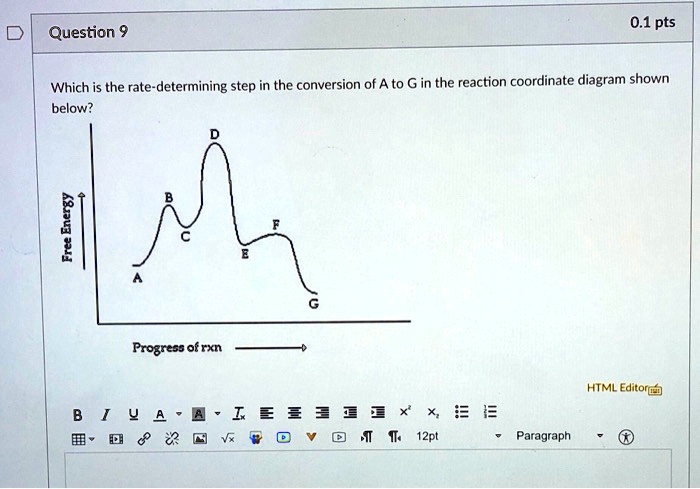
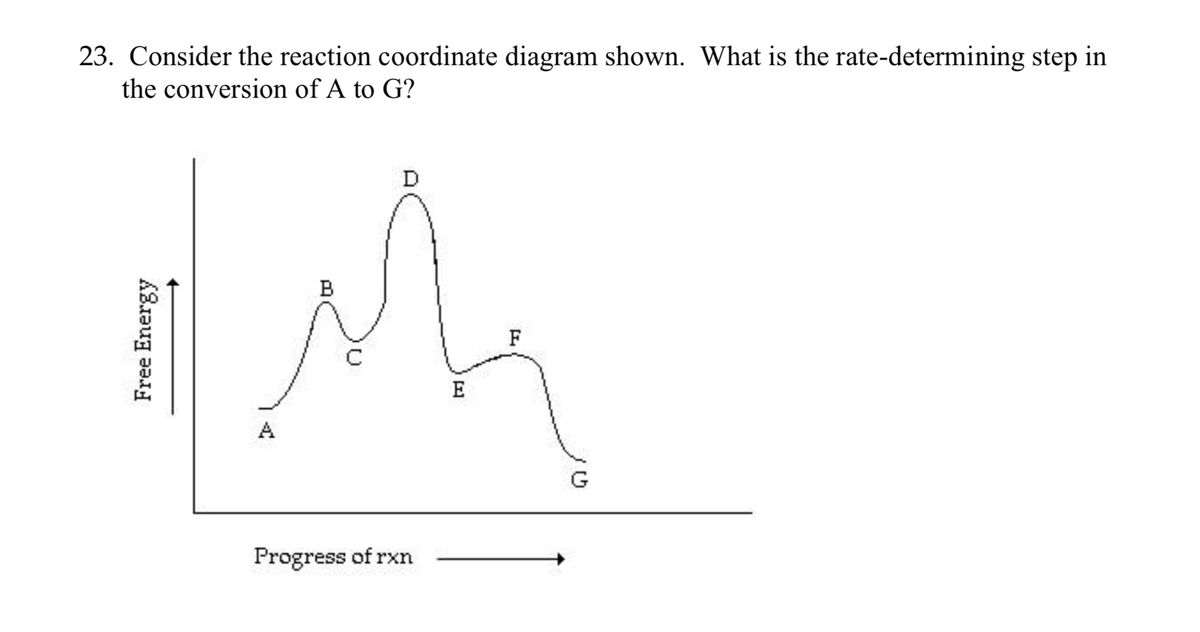
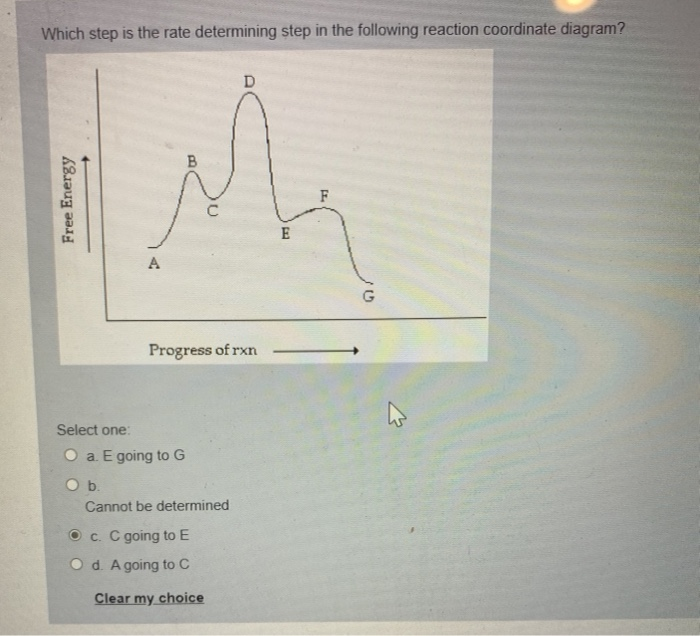
Solved Question 9 0 1 Pts Which Is The Rate Determining Step In The Conversion Of A To G In The Reaction Coordinate Diagram Shown Below 1 Progress Ofrxn Html Editorca 4 I 0 12pt Paragraph






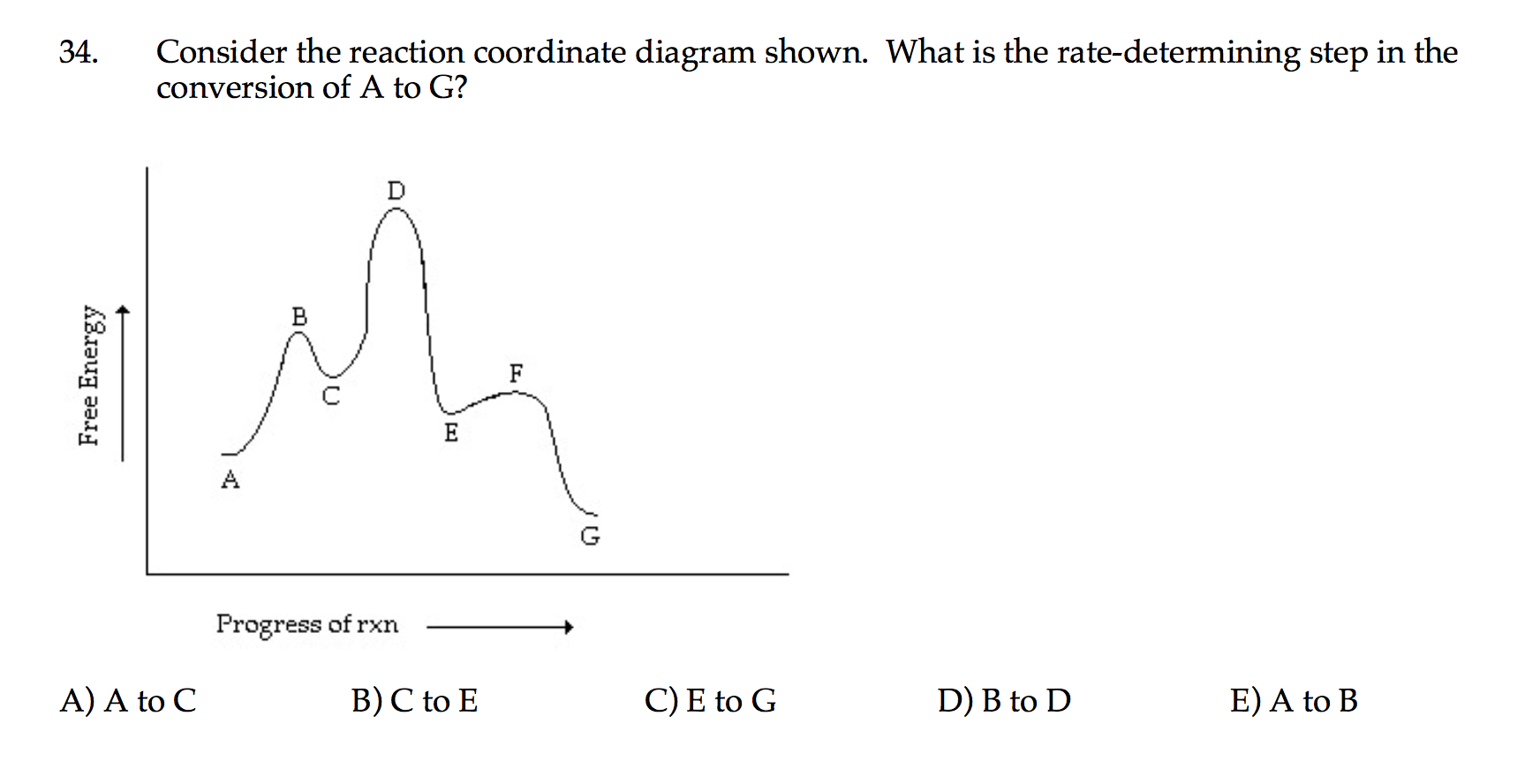








Comments
Post a Comment