41 beryllium bohr model diagram
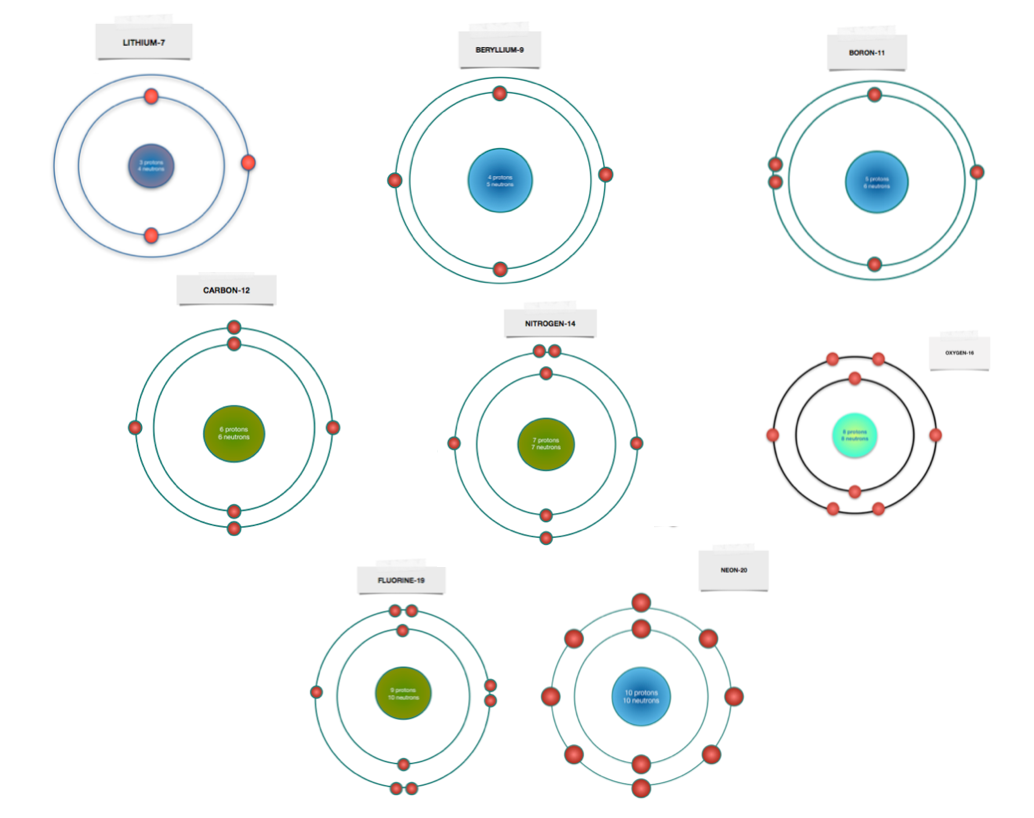
Use the information provided for each element to draw Bohr Model diagrams. Rather than drawing ... Beryllium - atomic #: 4, # of n: 5.2 pages Drawing Dohr model diagrams 1. Refer to the Bohr model chart on page 32 to help you complete the following table. Some answers are provided for you. (Hint: Remember that the maximum number of electrons in the first three shells is 2, 8, and 8.) Number of electrons 10 10 10 14/ 18 18 Number of electron shells Atom/ion neon atom fluorine atom
Then, here is the solution you are looking for. Now, you do not need to roam here and there for

Beryllium bohr model diagram
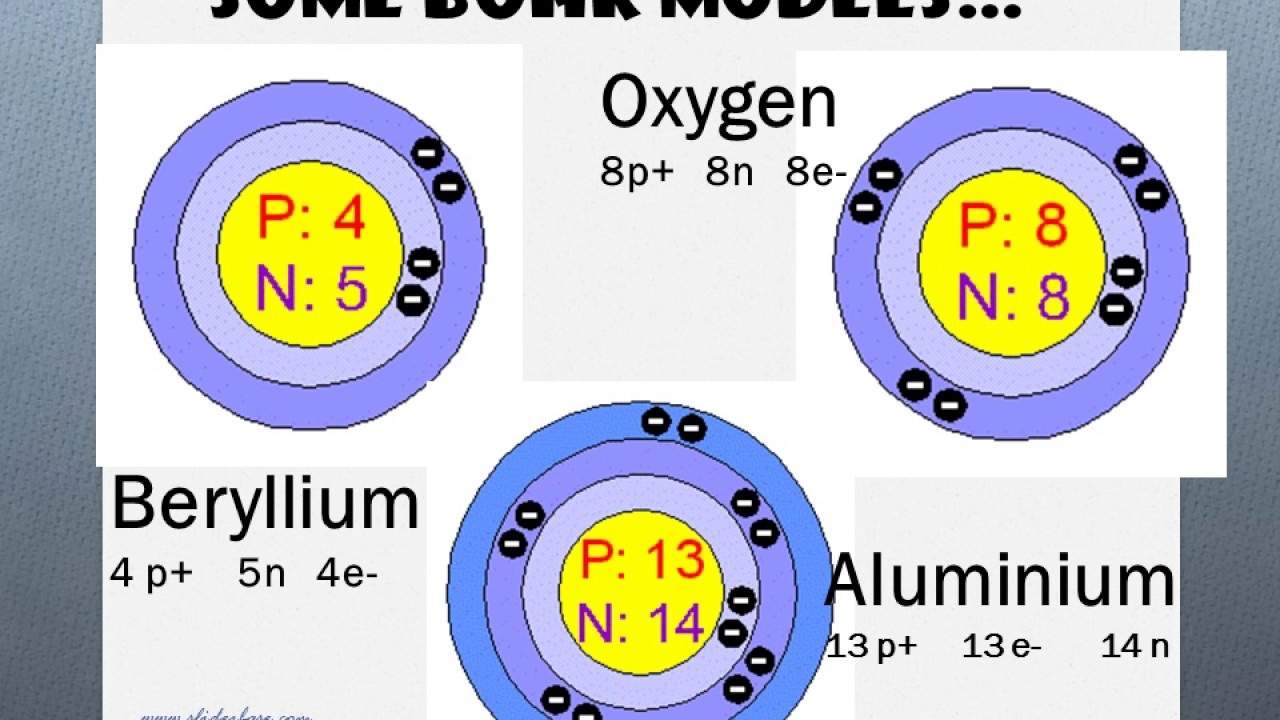
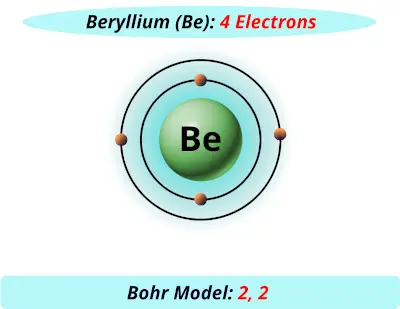
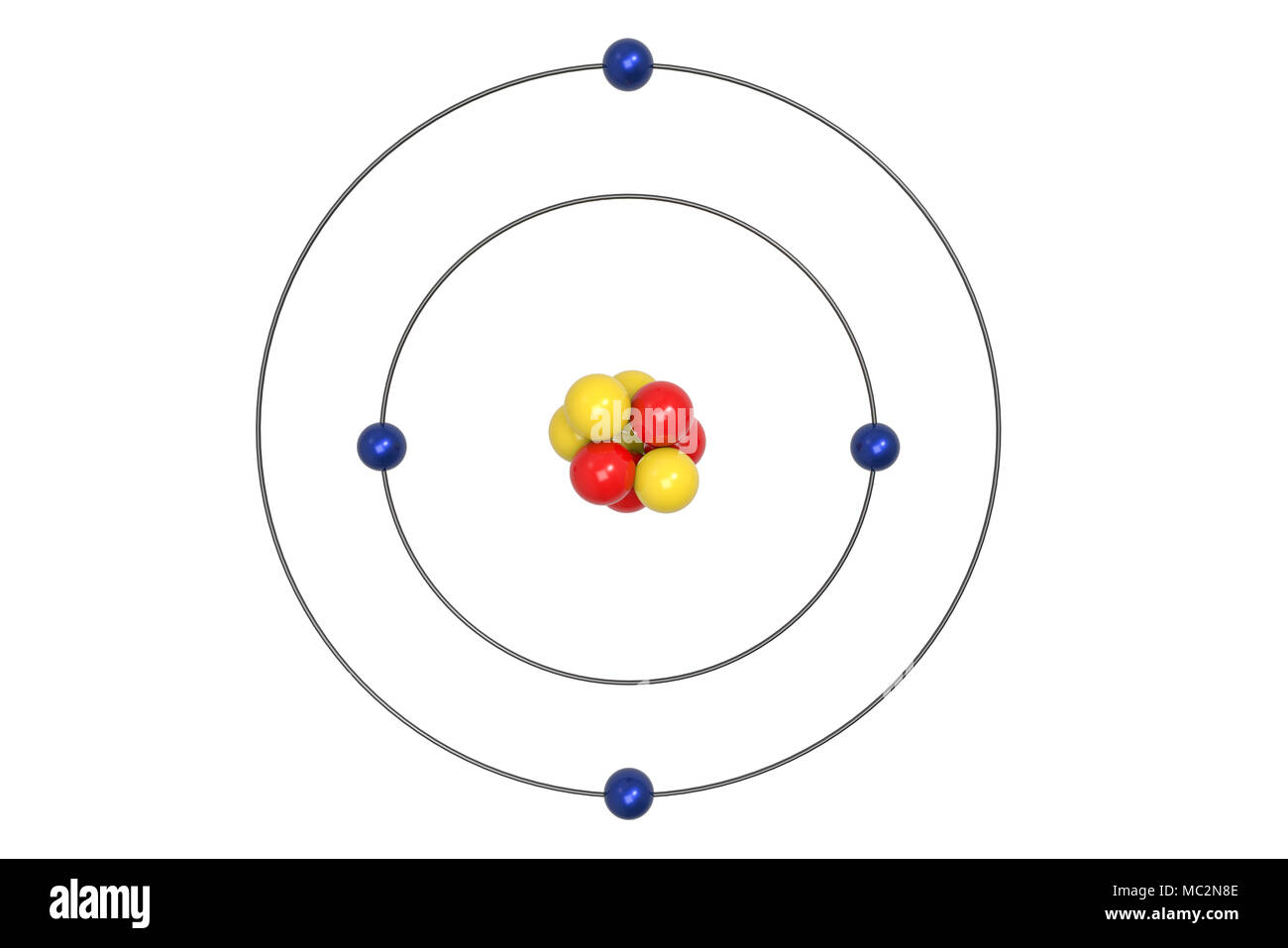
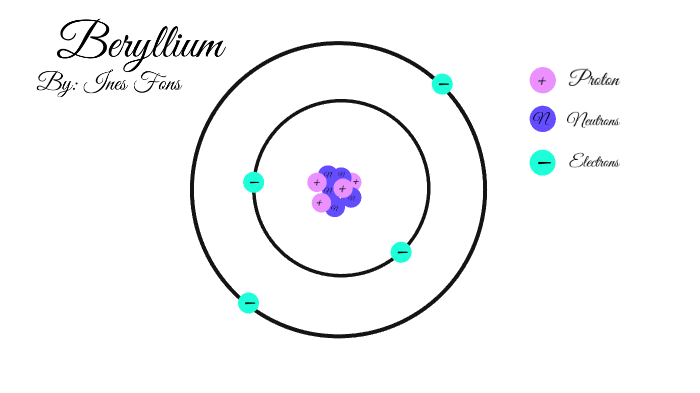
How To Draw A Bohr Model Of Atom. Jan 18, · aluminum, al bohr diagram. Draw a bohr model of this atom the atom has 17 protons, 18 electrons and 20 neutrons. From the periodic table, find the element, and identify its atomic number and atomic. Bohr diagrams 1) add the electrons. The Bohr model of Helium is drawn with only one electron shell and it contains 2 electrons. Helium is neutral and its atomic number is 2, hence, the number of protons and electrons available for its Bohr diagram is also 2. The number of neutrons for the Bohr diagram of Helium can be found by subtracting the number of protons from the atomic ... The Bohr Model for Beryllium (Be) has 4 protons in the nucleus due to the atomic number of Be being 4. ... (Mass number = protons + neutrons, 9 = 4 + n). Beryllium has four electrons to balance the four protons. The 4 electrons are arranged with 2 electrons in the first orbital and 2 electrons in the second orbital.
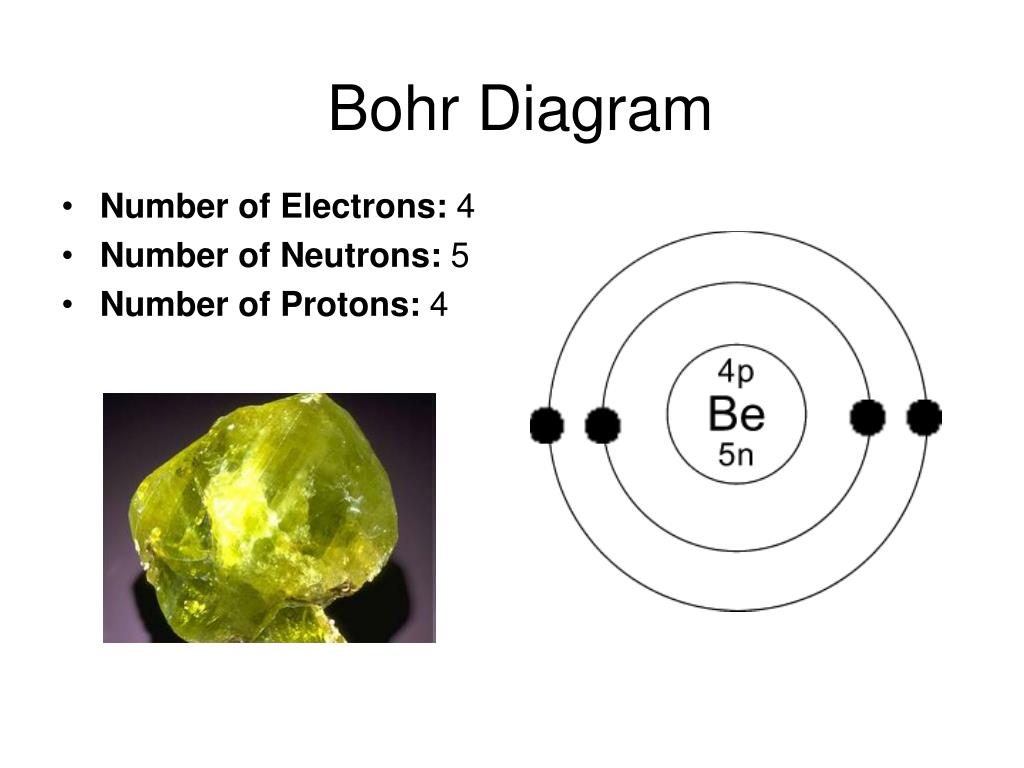
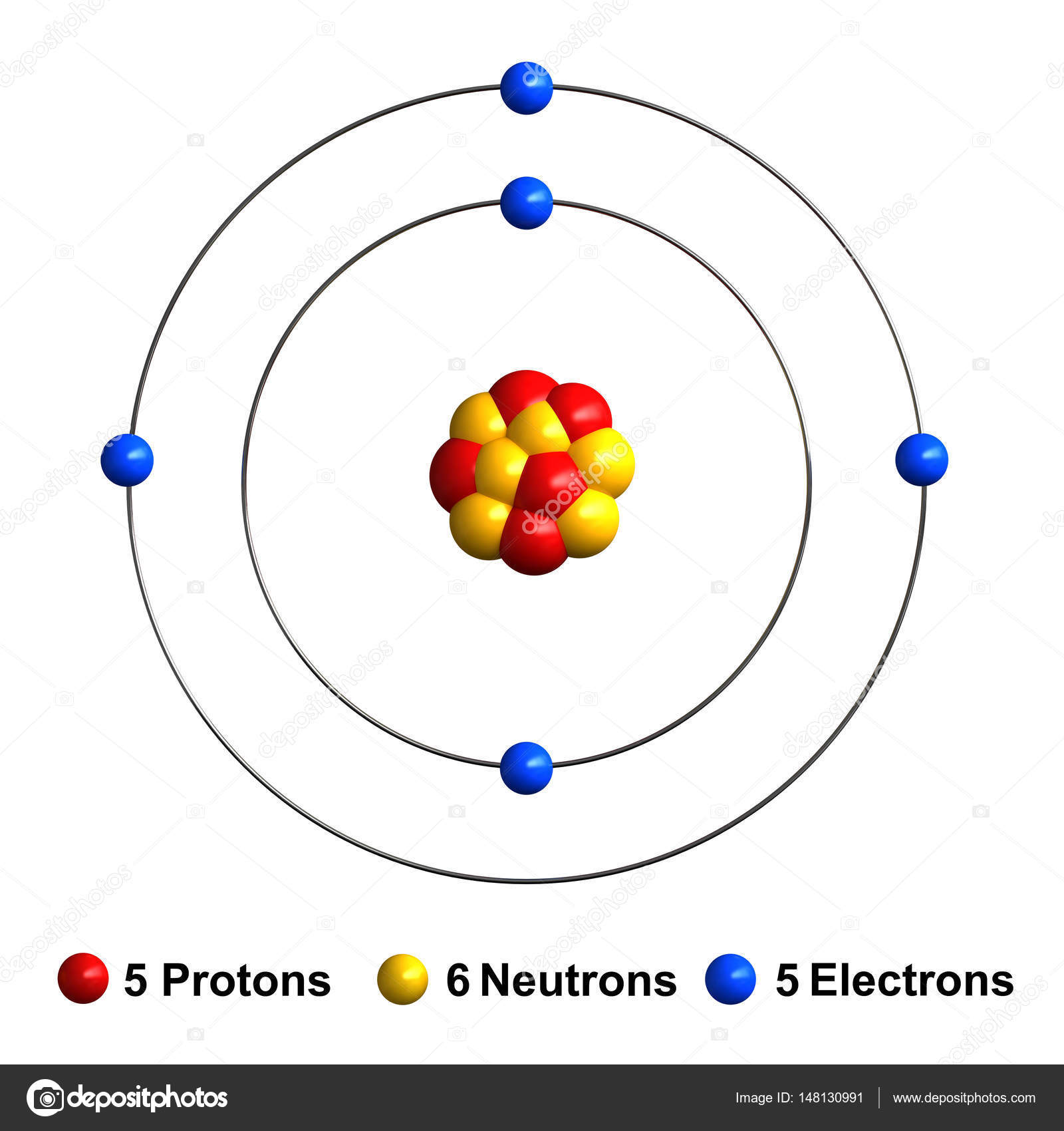
Beryllium bohr model diagram. Oct 28, 2018 · Calculate the number of protons, neutrons & electrons for the following. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. Atomic physics Bohr model of the atom. by crator- Bohr And Quantum Mechanical Model of Atoms Bohr model and the quantum mechanical model. This means the beryllium atom has four protons and four electrons. The Bohr Model for Beryllium (Be) has 4 protons in the nucleus due to the atomic number of Be being 4. The Mass number is 9 which means Beryllium needs 5 neutrons in the nucleus. (Mass number = protons + neutrons, 9 = 4 + n). Beryllium has four electrons to balance the four protons. The 4 electrons are arranged with 2 electrons in the first orbital and 2 electrons in the second orbital. Name: Beryllium Symbol: Be Atomic Number: 4 Atomic Mass: 9.012182 amu Melting Point: 1278.0 °C (1551.15 K, 2332.4 °F) Boiling Point: 2970.0 °C (3243.15 K, 5378.0 °F) Number of Protons/Electrons: 4 Number of Neutrons: 5 Classification: Alkaline Earth Crystal Structure: Hexagonal Density @ 293 K: 1.8477 g/cm 3 Color: gray Atomic Structure Answer to Class Example - Bohr Electron Configuration Drawing for Beryllium after it has satisfied the Octet Rule in Lesson 1.4
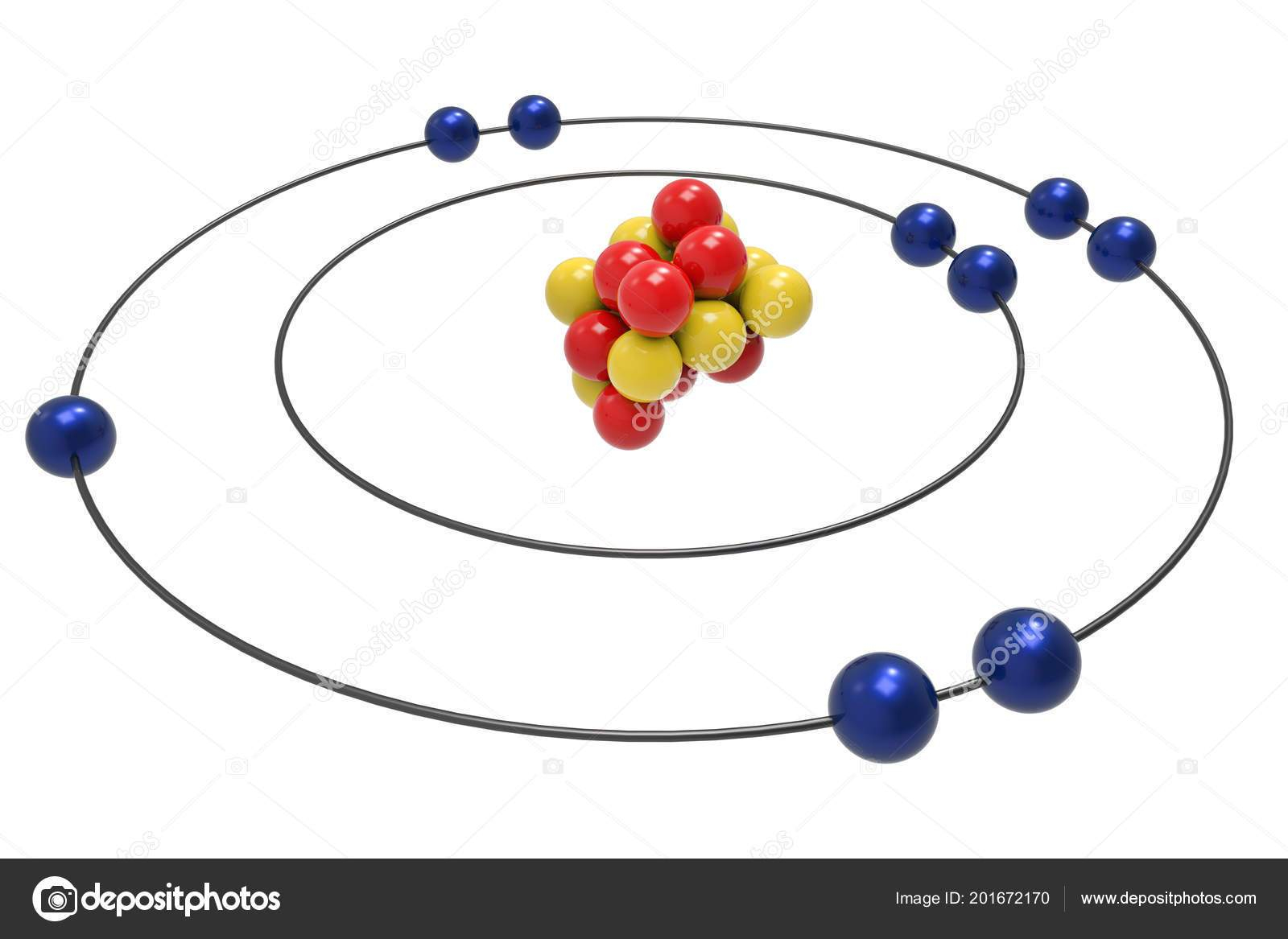
i How to Draw Bohr Diagrams : Dbetermine the number of. Prators and electrons [Same as anomic number in a nevtral. Com. 2)Determine the number of neurons.8 pages Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to ... Bohr model of Hydrogen (H) 1: 2: Bohr model of Helium (He) 2: 3: Bohr model of Lithium (Li) 2, 1: 4: Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11 ...
How do you draw Bohr model diagram of Beryllium atomic? Wiki User. ∙ 2009-09-18 00:54:37. Study now. See answer (1) Best Answer. Copy. Sulfur –. Presentation on theme: "Draw a Bohr Model of Beryllium Draw a Bohr Model of Diagram Rules 19 Bohr Model & Lewis Dot Diagram Practice 20 9/26 Warm up. File:Electron shell 004 Beryllium - no label.svg. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. Bohr Model Worksheet With Answers Fill Online Printable Bohr Model Physical Science Middle School Worksheets. The following drawings are bohr models for a beryllium fluorine and carbon atom. Bohr Model Diagram Cards Bohr Model Super Teacher Worksheets Teaching Chemistry All things algebra answer key unit 8.Bohr model drawing worksheet answer key. Download our FREE eBook guide to learn how, with the help of walking aids like canes, walkers, or rollators, you have the opportunity to regain some of your independence and enjoy life again.
What is beryllium Bohr diagram? The Bohr Model for Beryllium (Be) has 4 protons in the nucleus due to the atomic number of Be being 4. ... (Mass number = protons + neutrons, 9 = 4 + n). Beryllium has four electrons to balance the four protons. The 4 electrons are arranged with 2 electrons in the first orbital and 2 electrons in the second orbital.
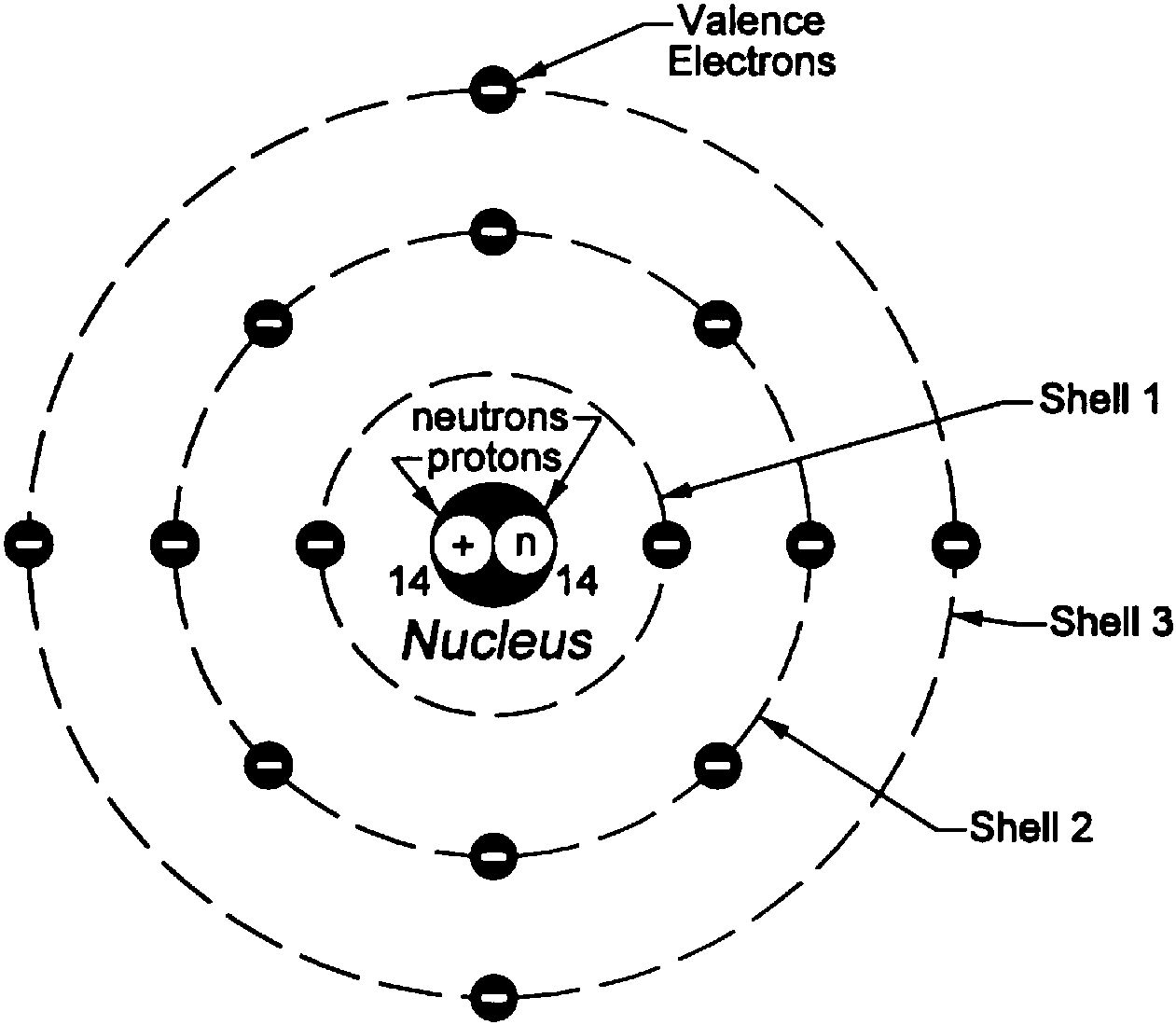
Bohr Model Worksheet Use the description sheet and the periodic table to help you complete the following Bohr models. 1. How many electrons can each shell hold? a. 1st = _____ b. 2nd = _____ c. 3rd = _____ Element Atomic # Atomic Mass Protons Neutrons Electrons Bohr Model Carbon 6 12 6 6 6 Hydrogen 1 1 Lithium 3 3 3
A bohr diagram can be used to visually show the bohr model of a particular atom. How to draw a bohr model for helium. Write the number of protons for the nucleus, 1p +. Give an example of an atom and an ion and draw a bohr rutherford. Bohr model of boron (b) 2, 3: Bohr model of beryllium (be) 2, 2: Bohr diagrams 1) add the electrons.
How do you draw Bohr model diagram of Beryllium atomic April 10th, 2019 - A Bohr Atom Model consists of a CORE of 16 Protons and 16 Neutrons 32 total AND 16 total Electrons in the Energy Levels the inner most level has 2 the second level has 8 and the third level New Bohr model Beryllium Be BIGLOBE
Beryllium Bohr Model Diagram. Feb 19, Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. Apr 24, The isotope beryllium-9, with five neutrons, is the stable form of the atom. Creating a 3D model provides a child with a visual representation of a.
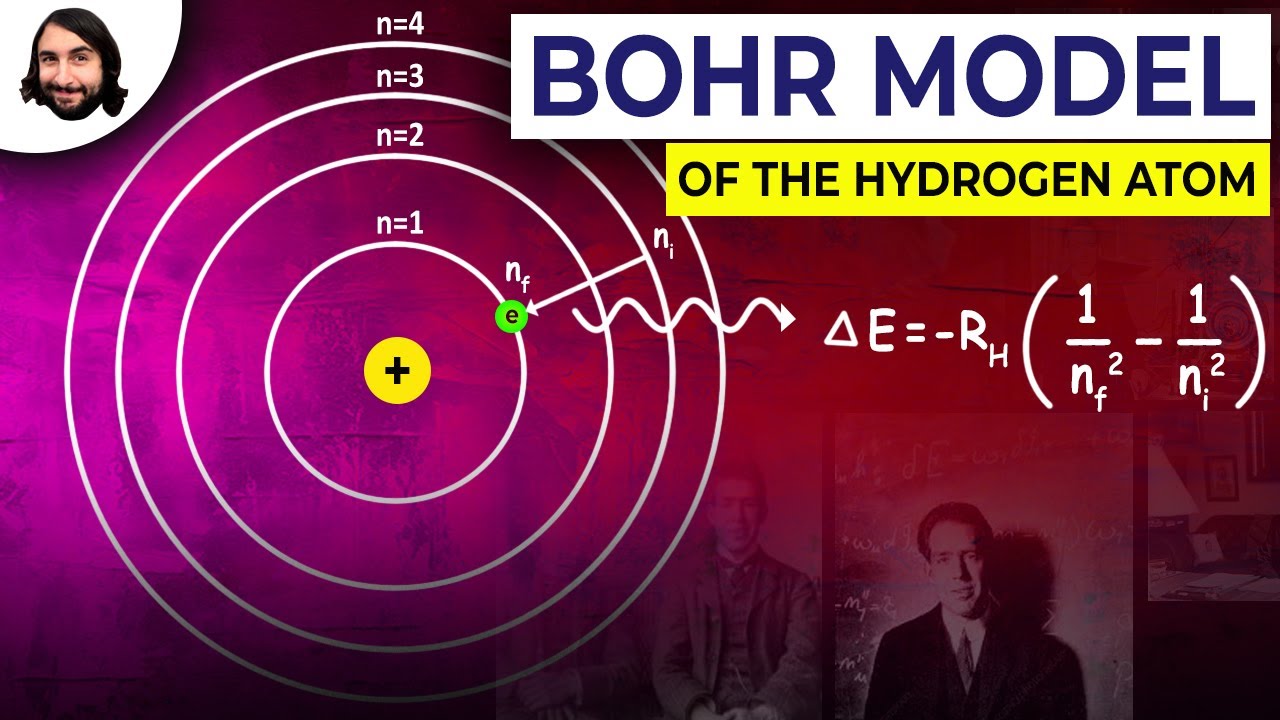
May 19, 2019 · A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Beryllium bohr diagram is in fact among images libraries inside our highest pictures gallery. I wish you're going to enjoy.
Steps to draw the Bohr Model of Beryllium atom — Steps to draw the Bohr Model of Beryllium atom. 1. Find the number of protons, electrons, and ...Total valence electrons in Beryllium: 2Electron in the First shell(K): 2Electrons in the Second shell(L): 2Number of electrons: 4Steps to draw the Bohr Model... · Find Valence electron of...
Mar 28, 2019 · Our Bohr model has succeeded in expressing the Beryllium (ion) correctly in the ionization energy. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. Number of Protons/Electrons: 4. New Bohr model Beryllium (Be) Number of Neutrons: 5.
Lewis Dot Diagrams… Gilbert Lewis used a different model than Bohr, and he only showed the valence e- in it. His model is called the . Lewis dot structure . He put dots around the symbols so that we can . see. just the . valence electrons . for the elements (so we can easily see which e- are going to react)
The Bohr Model for Beryllium (Be) has 4 protons in the nucleus due to the atomic number of Be being 4. ... (Mass number = protons + neutrons, 9 = 4 + n). Beryllium has four electrons to balance the four protons. The 4 electrons are arranged with 2 electrons in the first orbital and 2 electrons in the second orbital.
The Bohr model of Helium is drawn with only one electron shell and it contains 2 electrons. Helium is neutral and its atomic number is 2, hence, the number of protons and electrons available for its Bohr diagram is also 2. The number of neutrons for the Bohr diagram of Helium can be found by subtracting the number of protons from the atomic ...

“Fashion is part of the daily air and it changes all the time, with all the events. You can even see the approaching of a revolution in clothes. You can see and feel everything in clothes.†—Diana Vreeland
How To Draw A Bohr Model Of Atom. Jan 18, · aluminum, al bohr diagram. Draw a bohr model of this atom the atom has 17 protons, 18 electrons and 20 neutrons. From the periodic table, find the element, and identify its atomic number and atomic. Bohr diagrams 1) add the electrons.












Comments
Post a Comment