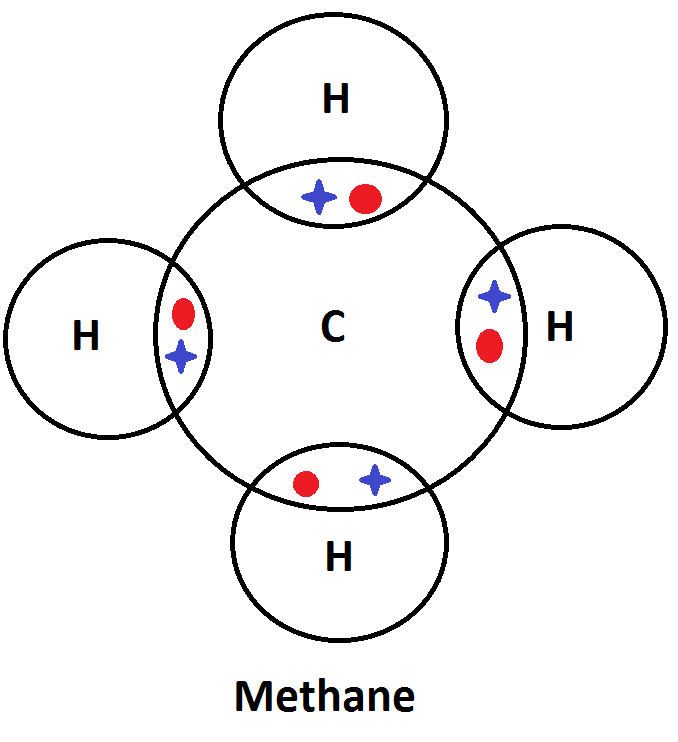
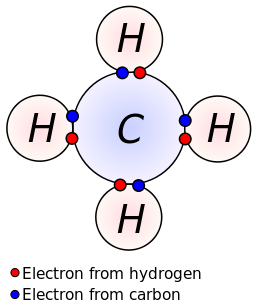
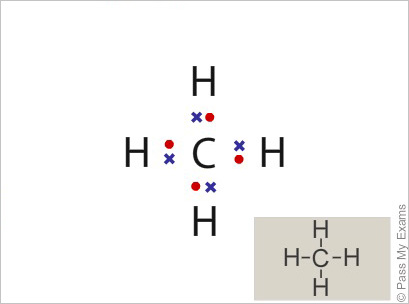
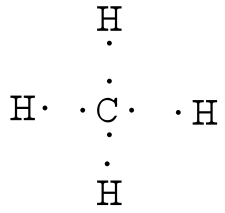
42 electron dot diagram for methane
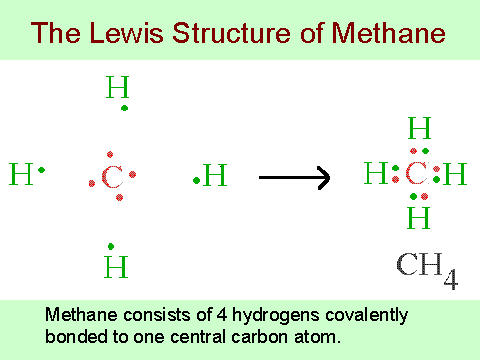
Methane (CH 4) Molecule Lewis Structure. Methane lewis structure contains four C-H bonds. There are no lone pairs in the valence shells of carbon atom. Carbon atom is the center atom and it is very easy to draw CH4 lewis structure. CH 4 lewis structure. There are following specifications in the lewis structure of methane. Steps to draw the lewis dot structure of BrCN. In cyanogen bromide, a carbon atom forms a triple bond with a nitrogen atom (cyanogen group) and a single covalent bond with bromine, a halogen. Step 1: First, the skeleton structure of the compound must be drawn, using the symbol of the elements and joining them by single bonds only.
In methane (CH4), each hydrogen atom shares one electron with carbon. ... What does the Lewis dot diagram represent? Answers: ... The mixing of electrons in the electron sea model to account for ...
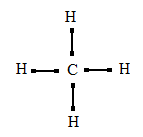
Electron dot diagram for methane
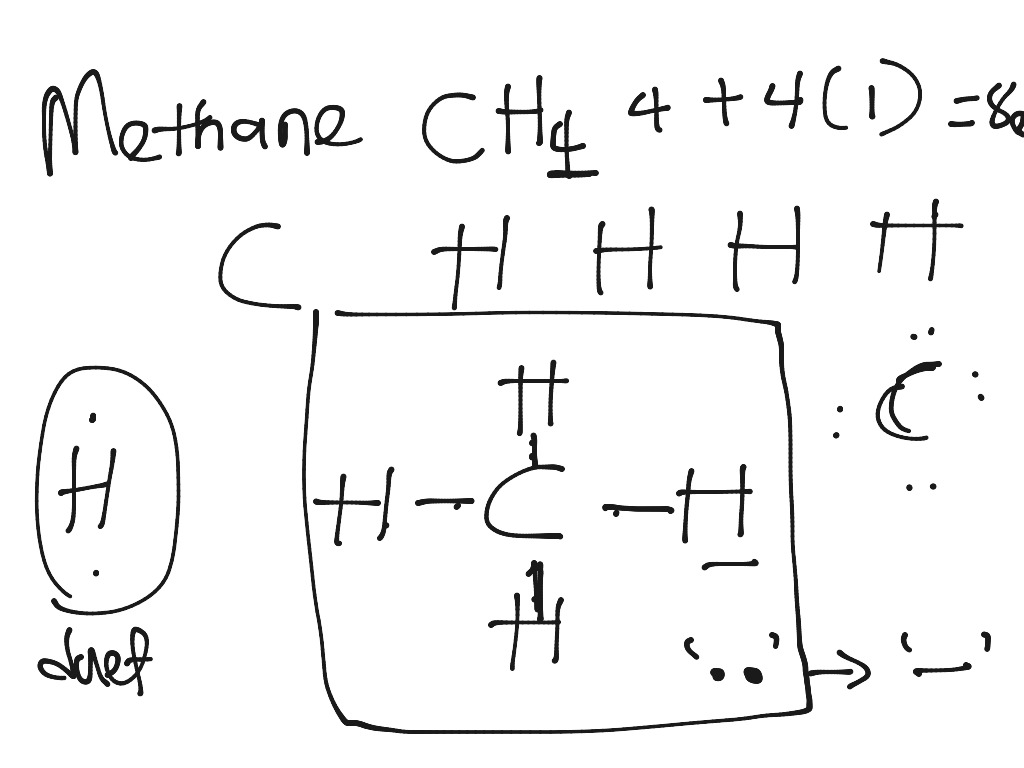
How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use... give the a electron dot diagram of i magnesium chloride ii nitrogen iii methane b molecular structure of i magnesium chloride ii nitrogen iii methane - Chemistry - TopperLearning.com | vorl6bx44 PRE-LAB QUESTIONS 1. Draw an electron dot diagram for methane. 2. Use Figure 9 and the periodic table (Figure 2) to construct a hypothesis about the melting points of stearic acid and sodium chloride. How will they compare, and how does that relate to the bonds that hold atoms in the compounds together? Figure 9: Structure of Stearic Acid Because of the statement that more that bonds are ...
Electron dot diagram for methane. Founded in 2002 by Nobel Laureate Carl Wieman, the PhET Interactive Simulations project at the University of Colorado Boulder creates free interactive math and science simulations. PhET sims are based on extensive education research and engage students through an intuitive, game-like environment where students learn through exploration and discovery. Ch4 Electron Dot Diagram. I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the. Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . CH4 Lewis Structure, Hybridization, Molecular Geometry, Bond Angle and Shape. Methane is one of the simple organic molecules, given its straightforward structure. It has the chemical formula of CH4 and comprises one carbon atom forming bonds with four hydrogen atoms. The compound is one of the main constituents of natural gas. Electron ionization is widely used in mass spectrometry, particularly for organic molecules. The gas phase reaction producing electron ionization is + + + where M is the atom or molecule being ionized, is the electron, and + is the resulting ion. The electrons may be created by an arc discharge between a cathode and an anode.. An electron beam ion source (EBIS) is used in …
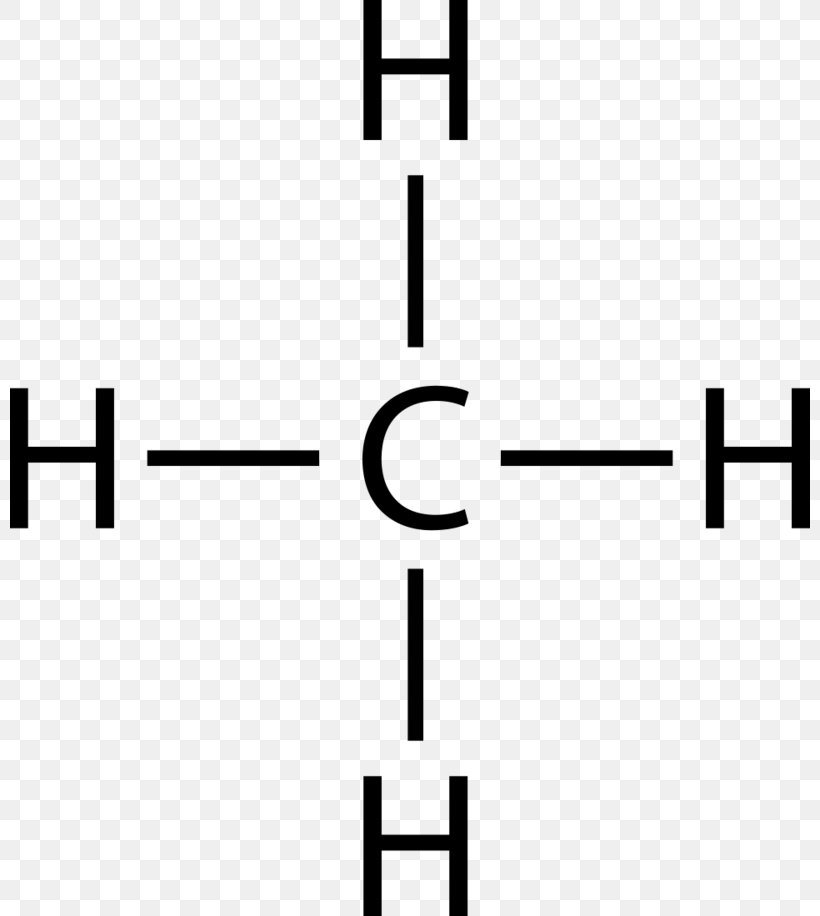
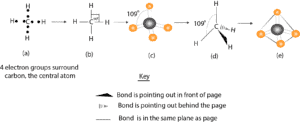
17. Acronyms and Abbreviations 733 BLS. . . . . . . . . .Bureau.of.Labor.Statistics BMI. . . . . . . . . .Bismaleimide BOGS. . . . . . . .Blade.Outer.Gas.Seal Dr. B. explains how to draw the Lewis dot structure for CH 4 (methane). The CH 4 Lewis Structure is one of the most frequently tested Lewis Structures.. Note that hydrogen atoms always go on the outside of a Lewis dot structure. This is because they can share a maximum of two electrons. Electron dot structure of non-polar covalent molecules on the basis of duplet and octet of electrons (example: hydrogen, chlorine, oxygen, nitrogen, carbon tetrachloride and methane. • Polar Covalent compounds based on – difference in electronegativity: Examples – HCl, NH. 3. and H. 2. O including electron dot structures. • Drawing C is a Lewis electron dot structure for methane. Figure 1 Alternative Representations of Methane. Lewis Electron Dot Structures. Figure 2 animates the rules for drawing a Lewis electron dot structure using C 2 H 6 as an example. Click within the figure to view the animation.
Answer: I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the middle is the one Carbon, and the four yellow ones are Hydrogens. Now I have drawn the Valence Electrons. These are the blue dots next to the... Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Jan 19, 2022 · It is also known as an electron dot structure where each bond is shown as two dots between two atoms. Let us consider the Lewis structure of benzene step by step- Step 1 – The first step is the determination of the total no. of valence electrons of each atom present in benzene (C6H6), i.e; carbon and a hydrogen atom . Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. It is important to remember that Lewis valence dot diagrams are models that Methane is the main component of natural gas, and its chemical formula is CH4.
The key difference between Lewis dot symbol and Lewis structure is that Lewis dot symbol represents electrons in the outermost electron shell of an atom in a molecule whereas Lewis structure represents the structure of molecules using symbols for chemical elements and dot symbols.. Lewis structure is a simple structure which represents the chemical bonds and lone electron pairs in simple ...
Methane is a one-carbon compound in which the carbon is attached by single bonds to four hydrogen atoms.It is a colourless, odourless, non-toxic but flammable gas (b.p. -161℃). It has a role as a fossil fuel, a member of greenhouse gas and a bacterial metabolite.
2 days ago · Step 3: Sketch the Skeletal Diagram of the Molecule. In Lewis Structure, we use atomic symbols like C for carbon, H for hydrogen to represent the constituent atoms, and electron dot notation to represent the valence shell electrons. Let us look at the below skeletal sketch: The atomic symbols: Atomic symbols along with dot notations:
Drawing the Lewis Structure for F. 2 F2 is a reddish gas at room temperature. The F2 Lewis structure is similar to Br2, Cl2, and I2 since F, Br, Cl, and I are all in Group 7 and have 7 valence electrons. For the F2 Lewis structure there are a total of 14 valence electrons available. What type of bonding does methane have? hydrogen covalent bonds
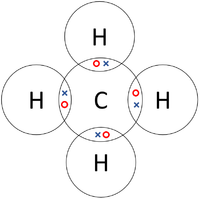
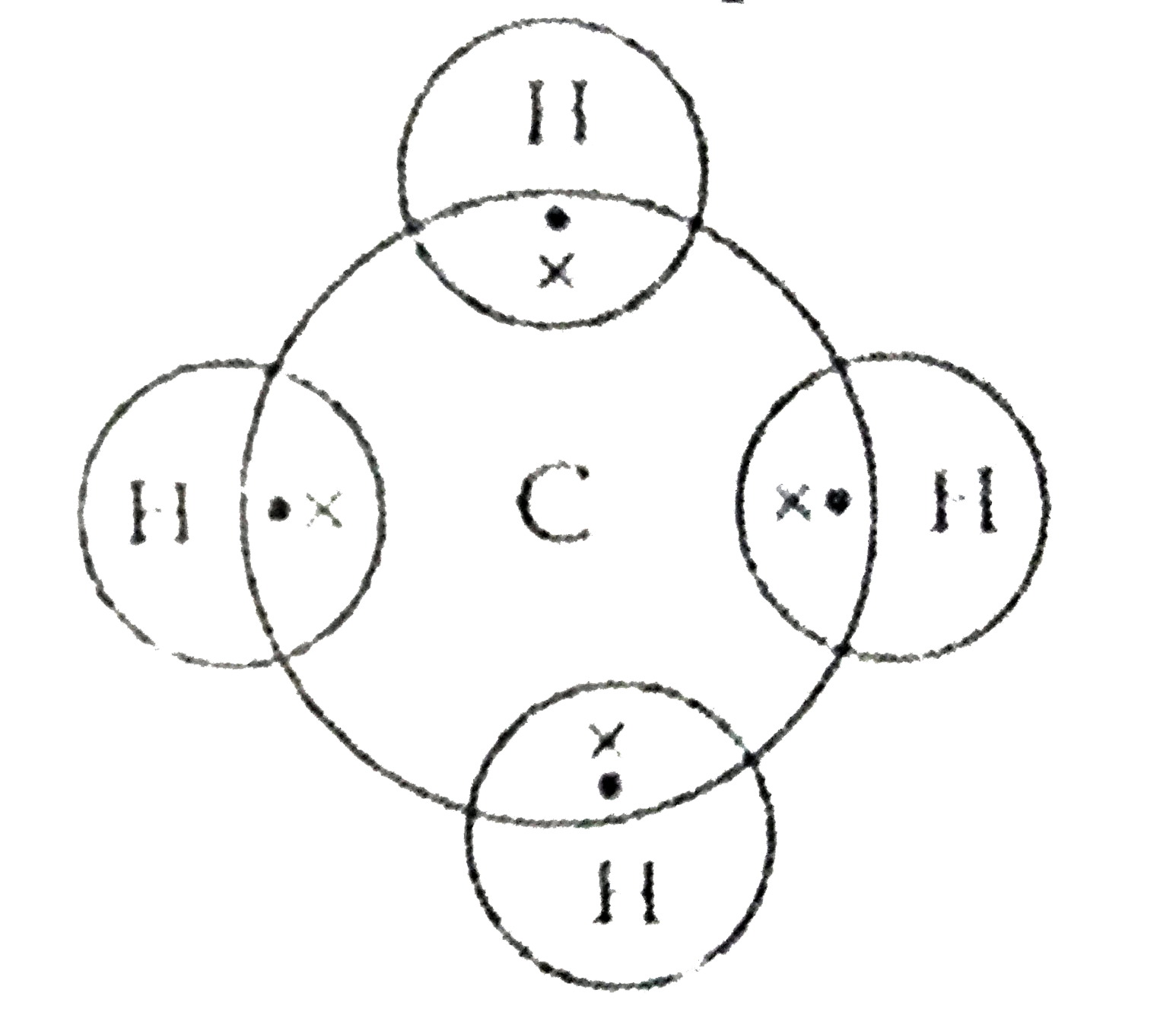
Electron dot structure of methane is- (Image attached) The covalent bonds are present in between four hydrogen atoms and the single carbon atom at the centre of the molecule. (i) Methane is a poor conductor of electricity because in methane all bonds are covalent bonds and therefore no free electrons are present in the molecule that can help in ...
Electron dot structures or Lewis dot formula - It defines the nature of bond and position of atoms of the molecule which are connected in the molecule. Electron dot structures of carbon dioxide. The carbon is the central atom of this molecule. Oxygen atom contains 6 valence electrons which form 2 lone pairs.
Vor 2 Tagen · Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step. Firstly, look for the total number of valence electrons required by a single CH4 molecule, which is sixteen. Next, a search of electrons is required by a single CH4 molecule to reach a stable condition. It is eight for a single CH4 molecule, as four are needed by the …
The total valence electron available for drawing the Methane (CH4) lewis structure is 8. The steric number of the central atoms in methane is 4 that ensures that it has an Sp3 hybridization.
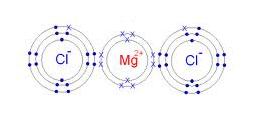
1)Draw an electron-dot diagram for each of the following substances: a CaO(an ionic compound) b HBr c N2 Base your answers to questions 2 and 3 on the information below and on your knowledge of chemistry. The formulas and the boiling points at standard pressure for ethane, methane, methanol, and water are shown in the table below.
View full document. PRE-LAB QUESTIONS 1. Draw an electron dot diagram for methane. 2. Use Figure 9 and the periodic table (Figure 2) to construct a hypothesis about the melting points of stearic acid and sodium chloride. How will they compare, and how does that relate to the bonds that hold atoms in the compounds together?
Methane is a colorless, odourless and highly flammable gas which is the main component of natural gas. Electron dot structure of methane is- (Image attached) The covalent bonds are present in between four hydrogen atoms and the single carbon atom at the centre of the molecule. What do you call CH4? Methane is a colorless odorless gas.
methane Ill:ethanol A. Draw the complete Lewis electron-dot diagram for methane, CH4, in the appropriate cell in the table above. [1 POINT] i. Would you classify methane as a polar or nonpolar olecule? Justify your answer. [1 POINT] -there IS , B. Which of the four molecules contains the shortest carbon-to-carbon bond? Explain. [1 POINT] q has e -
Find an answer to your question draw the electron dot structure of CH4.Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single schematron.org's one of the easier Lewis structures to draw.
Methane Lewis Structure. Here are a number of highest rated Methane Lewis Structure pictures upon internet. We identified it from honorable source. Its submitted by government in the best field. We say yes this nice of Methane Lewis Structure graphic could possibly be the most trending topic in the same way as we share it in google benefit or ...
Draw Electron - Dot Structure and Structural Formula of Methane.
Finally after a long discussion about the CO2 lewis structure or electron dot structure, molecular geometry, polarity, bond angle, etc. We are going to take the last overview of this article with the help of some bullet points. The net dipole moment of CO2 is zero. The electron and molecular geometry of CO2 are linear.
Which of the following is an acceptable Lewis Structure for the diatomic nitrogen molecule? ? ? ? ? In the correct Lewis structure for the methane (CH 4) molecule, how many unshared electron pairs surround the carbon? ? 0 ? 2 ? 4 ? 8; In the correct Lewis structure for water, how many unshared pairs of electrons will oxygen have? ...
Check me out: http://www.chemistnate.com
Electron Configuration 3. Orbital Diagram 4. Quantum Numbers 5. LEWIS SYMBOLS. LEWIS SYMBOLS 1. Electrons are represented as DOTS 2. Only VALENCE electrons are used Atomic Hydrogen is H • Atomic Lithium is Li • Atomic Sodium is Na • All of Group 1 has only one dot. The Octet Rule Atoms gain, lose, or share electrons until they are surrounded by 8 valence …
PRE-LAB QUESTIONS 1. Draw an electron dot diagram for methane. 2. Use Figure 9 and the periodic table (Figure 2) to construct a hypothesis about the melting points of stearic acid and sodium chloride. How will they compare, and how does that relate to the bonds that hold atoms in the compounds together? Figure 9: Structure of Stearic Acid Because of the statement that more that bonds are ...
give the a electron dot diagram of i magnesium chloride ii nitrogen iii methane b molecular structure of i magnesium chloride ii nitrogen iii methane - Chemistry - TopperLearning.com | vorl6bx44
How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
This image depicts Centers for Disease Control and Prevention (CDC) intern, Maureen Metcalfe, as she was using one of the agency’s transmission electron microscopes (TEM). The microscope’s screen was displaying a thin section of the variola virus, revealing some of the ultrastructural features displayed by this pathogenic organism, which is the cause of smallpox.
Produced by the National Institute of Allergy and Infectious Diseases (NIAID), this digitally colorized scanning electron microscopic (SEM) image depicts four, yellow colored, Group A Streptococcus (GAS), Streptococcus pyogenes bacteria, which were atop the surface of a human white blood cell (WBC), known as a neutrophil.
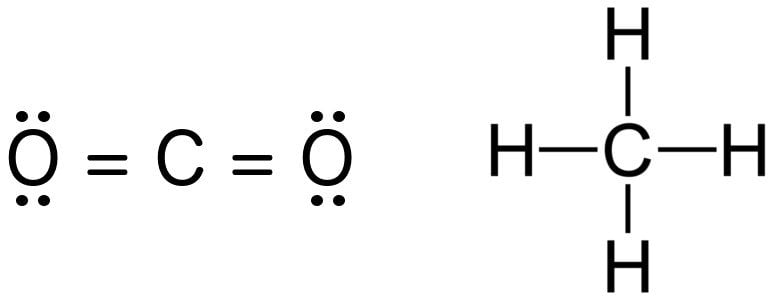

















Comments
Post a Comment