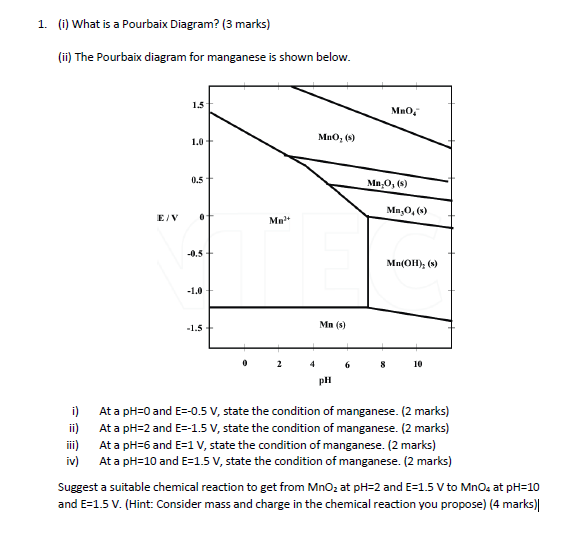
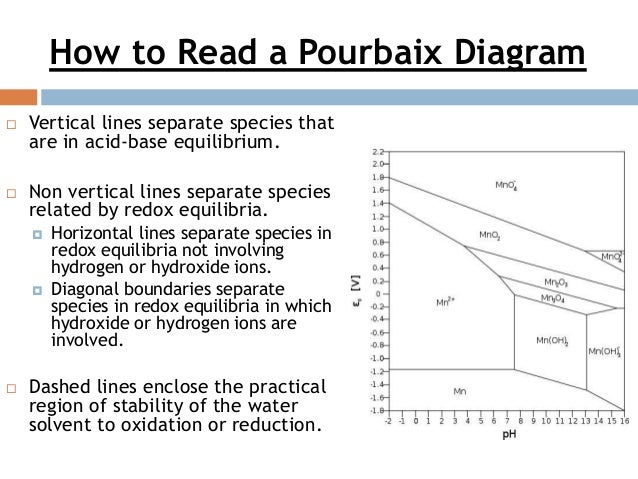
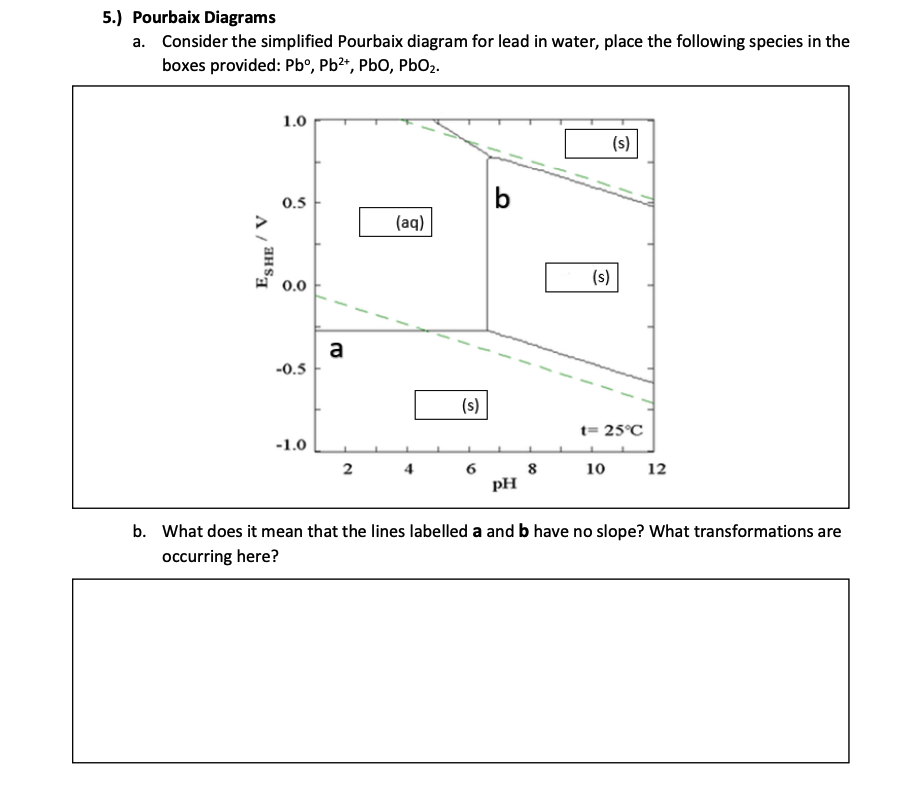
41 how to read pourbaix diagram
1 was fitted to the α 3 Y Pourbaix diagram with the following results: E 1/2 (pH 0) = 1.37 ± 0.02 V, ln(10)RT/nF = 59 ± 5 mV, pK rB = 6.7 ± 0.2, and pK oB = 7.4 ± 0.2. The fitted line is displayed in gray in Fig. 1 F and the fitting residuals are shown in the Inset. • Pourbaix diagrams are plotted in the axes Electrode potential of the metal vs. PH of the electrolyte. • Oxidizing conditions are described by the top part of the diagram (high positive electrode potential) • Reducing conditions are described by the bottom part of the diagram (high negative electrode potential). ... How to read Pourbaix ...
Jan 22, 2018 — A Pourbaix diagram provides information about the stability of a metal as a function of pH and potential. These diagrams are available for ...
How to read pourbaix diagram
How to read pourbaix diagram. Read a phase diagram and explain its importance Identify phase equilibrium lines, triple points and critical points on a phase diagram Describe at what point a substance is a supercritical fluid 4.6: Pourbaix Diagram s Last updated; Save as PDF Page ID 183315; No headers. March 22nd, 2019 - 3 1 How to read Pourbaix diagrams Pourbaix diagrams 1 also known as a potential pH diagrams show the possible stable equilibrium phases of a specific aqueous electrochemical system at a set temperature mostly at 298K Predominant ion boundaries are represented by lines As such a Pourbaix diagram can be read much like a ... Pourbaix diagrams are useful in predicting the sponta-neous direction of electrochemical reactions, identifying the corrosion products and predicting the changes in environment in terms of potential and pH that result in high or low corrosive attack [8]. The present work provides Pourbaix diagrams for chromium carbides that can be used
How to read pourbaix diagram. originator, Pourbaix (1963), a Belgium electrochemist and corrosion scientist. These diagrams represent the stability of a metal as a function of potential and pH. at a particular combination of pH and potential, a stable phase can be determined from the Pourbaix diagram. In such diagrams, the redox potential of the corroding system is 4.6: Pourbaix Diagrams Last updated; Save as PDF Page ID 183315; No headers. Pourbaix Diagrams plot electrochemical stability for different redox states of an element as a function of pH. As noted above, these diagrams are essentially phase diagrams that map the conditions of potential and pH (most typically in aqueous solutions) where different redox species are stable. Latimer and Frost diagrams help predict stability relative to higher and lower oxidation states, usually at one fixed pH. Pourbaix diagrams help understand pH-dependent equilibria, which are often coupled to solubility equilibria and corrosion (which will be talked about more later). Pourbaix diagrams are plotted in the axes Electrode potential of the metal vs. PH of the electrolyte. Oxidizing conditionsare described by the top part of the diagram (high positive electrode potential). Reducing conditionsare described by the bottom part of the diagram (high negative electrode potential).
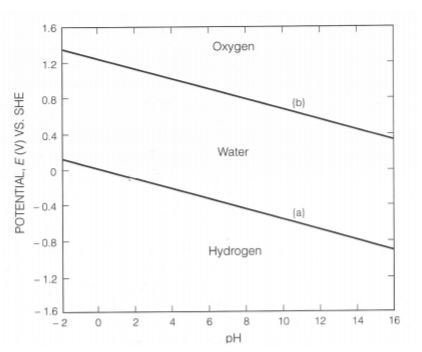
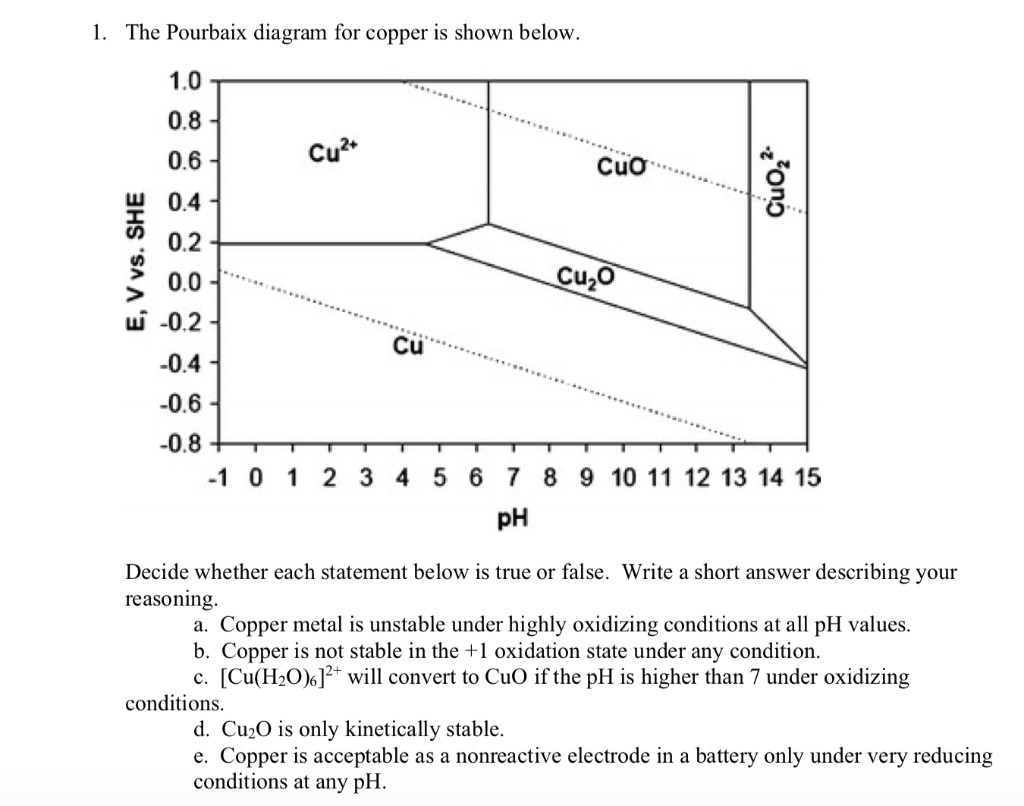
Pourbaix Diagrams For Copper 3/3 [PDF] [PDF] Pourbaix Diagrams For Copper This is likewise one of the factors by obtaining the soft documents of this pourbaix diagrams for copper by online. You might not require more epoch to spend to go to the book introduction as capably as search for them. Ellingham diagram was basically a curve which related the Gibbs energy value with the temperature. Gibbs energy is given as: ΔG = ΔH - TΔS. Where ΔH is the change in enthalpy and ΔS is the change in entropy. With respect to a reaction, Gibbs energy can be related to the equilibrium constant as: ΔG o = - RTlnK. Where K is the ... E-pH Diagram of. Water. The following example illustrates how the stability or predominance diagram of water can be constructed from its basic thermodynamic information. The following equationdescribes the equilibrium between hydrogen ions and hydrogen gas in an aqueous environment: ( reference) Adding sufficient OH - to both sides of reaction ... Pourbaix diagrams [1], also known as a potential/pH diagrams, show the possible stable equilibrium phases of a specific aqueous electrochemical system at a set temperature (mostly at 298K). Predominant ion boundaries are represented by lines. As such, a Pourbaix diagram can be read much like a standard phase diagram with a different set of axes ...
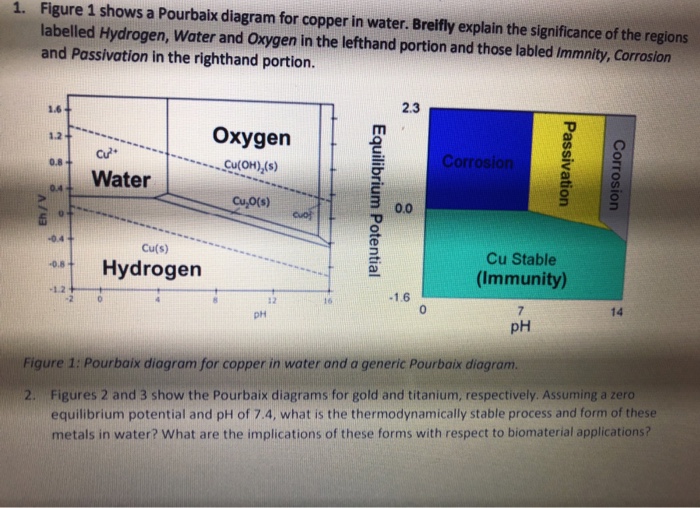
well as surface characteristics of materials. Eh-pH diagrams are thus essential to understanding solute and radionuclide transport in groundwater. The most well-known studies on comprehensive Eh-pH diagrams are those of Pourbaix (1966) and Brookins (1988). The former discussed corrosion, passivation and immunity of materials, while the These diagrams are available for over 70 different metals. Pourbaix diagrams have several uses, including in corrosion studies. A Pourbaix diagram is also known as a potential/pH diagram, equilibrium diagram, EH-pH diagram, a pE/pH diagram on an E-pH diagram. The Pourbaix diagrams (potential-pH diagrams) for nickel at 25- °C have been revised. Identify the forces acting on the loudspeaker and the n draw the m on the free body diagram. Objects do not always have four forces acting up on the m. By a box or a small circle and to draw the force arrow from the center of the box or circle outward in the directi on in which the force is acting.Call on e of the m scale 1 and the o the r scale 2. Identify the forces acting on the loudspeaker ... A Pourbaix diagram is a plot of the equilibrium potential of electrochemical reactions against pH. It shows how corrosion mechanisms can be examined as a function of factors such as pH, temperature and the concentrations of reacting species. Background Electrode potentials
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
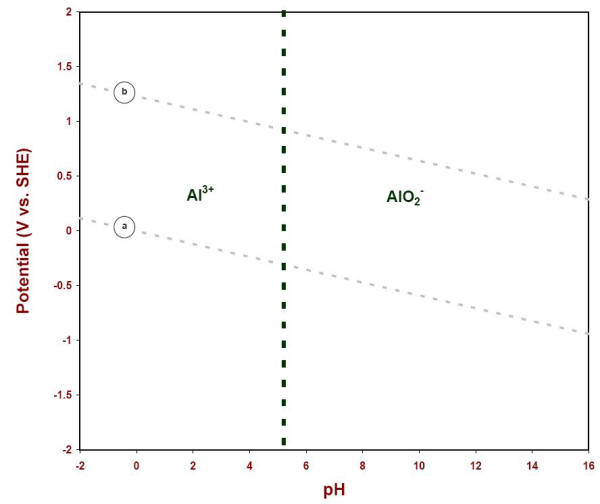
Eh pH diagrams are also known as Pourbaix Diagrams according to author of the famous Pourbaix diagram handbook 12 . The most simple type of these diagrams is based on a chemical system consisting of one element and water solution, for example, the Mn H 2O system.
devices to read Pourbaix Diagram - Materials Project Documentation A Pourbaix diagram, also frequently called a potenial-pH diagram, or E-pH diagram, is a representation of aqueous phase electrochemical equilibria. It is a two-dimensional representation of a three-dimensional free energy-pH-potential diagram. In other words, it shows water-stable
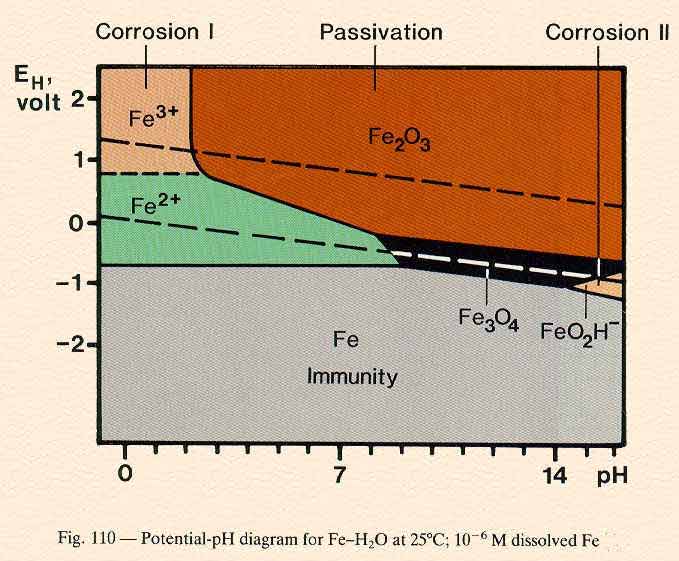
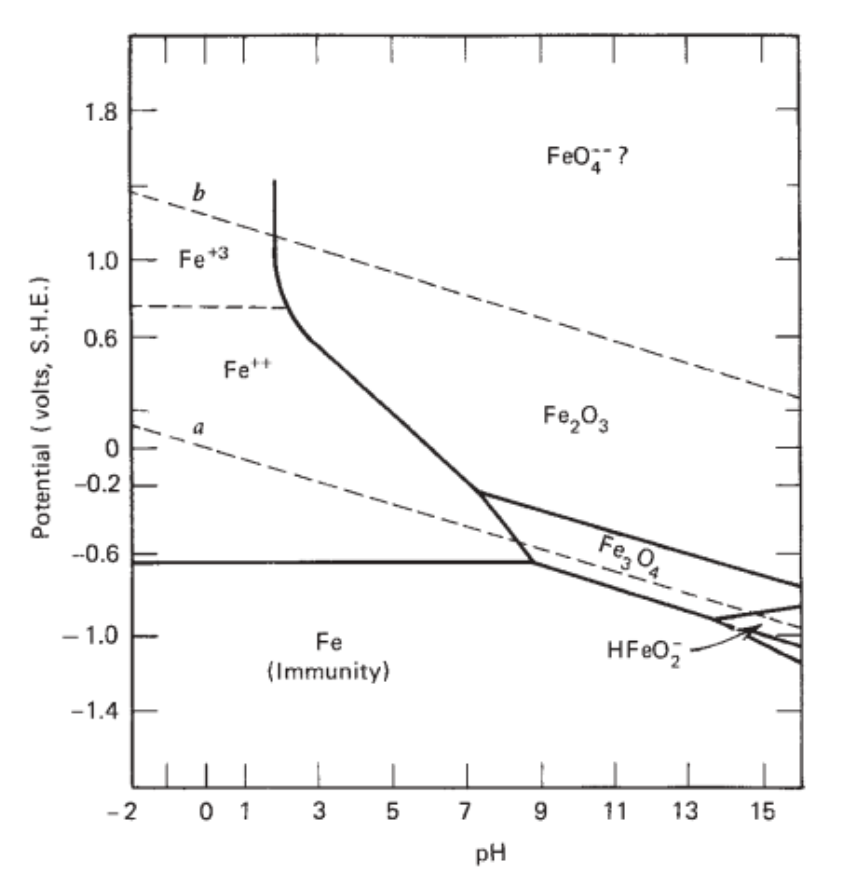
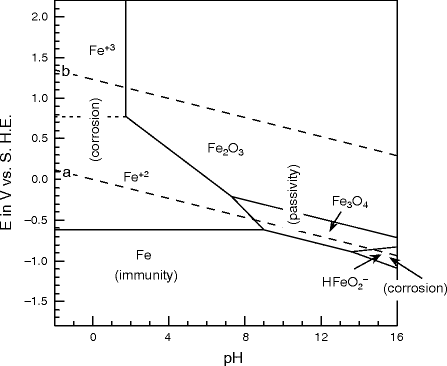
The Pourbaix diagram indicates the possibility of attack by solutions of pH values above ∼10.5, but the position of this limit is influenced by temperature, by the constitution of the solution, and by the surface condition of the metal. Corrosion will ensue if the surface oxide is significantly soluble, which will occur increasingly beyond pH 12.
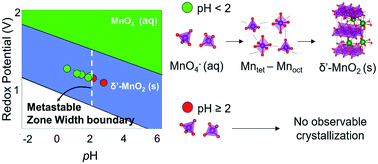
Kinetic Origins Of The Metastable Zone Width In The Manganese Oxide Pourbaix Diagram Journal Of Materials Chemistry A Rsc Publishing
As such a Pourbaix diagram can be read much like a standard phase diagram with a different set of axes. Similarly to phase diagrams, they do not allow for reaction rate or kinetic effects. Beside potential and pH, the equilibrium concentrations are also dependent upon, e.g., temperature, pressure, and concentration.

Pdf Construction And Verification Of Pourbaix Diagrams For Hydrogen Sulfide Corrosion Of Mild Steel Semantic Scholar
E - pH diagrams are also known as Pourbaix Diagrams, after the author of the famous Pourbaix diagram handbook1. The simplest type of these diagrams is based on a chemical system consisting of one element and a water solution, for example, the Mn-H 2O system. The system can contain several types of species, such as dissolved ions, condensed
radiating lines (upper left corner of the diagram). Now, with the straightedge running through these two points, read off the oxygen partial pressure (in atmospheres) where the straightedge crosses the "Po2" scale, and this is the equilibrium partial pressure.
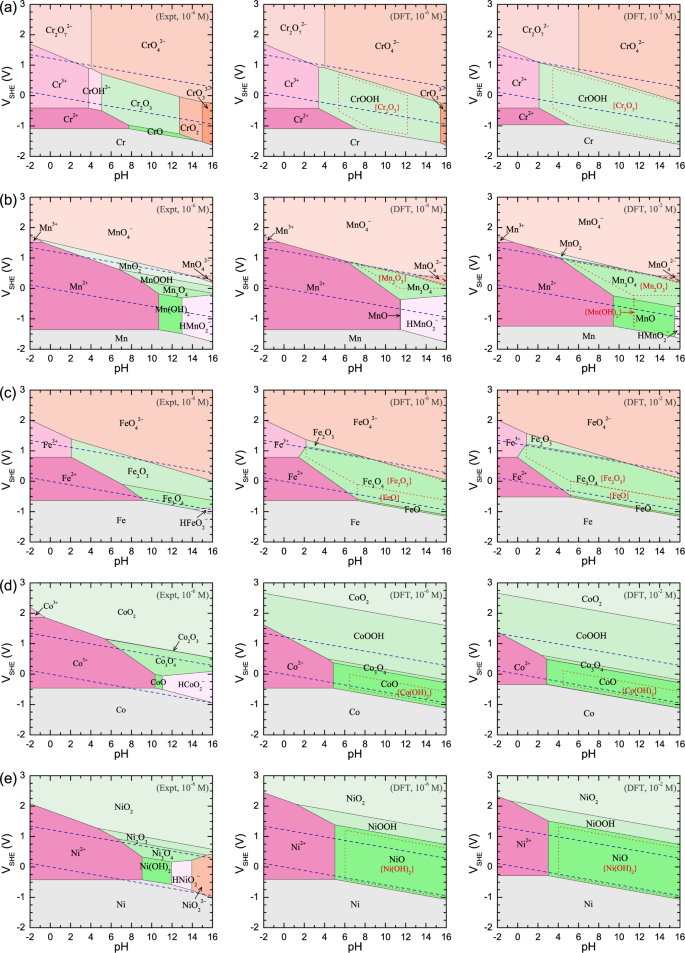
Reliable Electrochemical Phase Diagrams Of Magnetic Transition Metals And Related Compounds From High Throughput Ab Initio Calculations Npj Materials Degradation
For (11), we can read from the Pourbaix diagram that under standard conditions it will be going towards oxidization, which will pair with (9) to make a local net reaction. Normally the local concentration of H. 2. on the cathode is very low, so the line for (11) should actually
POURBAIX DIAGRAMS Phase diagrams for corrosion scientists! Nernst equation again… (different notation) ] 2 [] 1 [log 0 2.3] 2 [] 1 [0 ln 1 0 2 + + Δ =Δ + ↓ + + Mn Mn nF RT V V Mn Mn nF RT V V V The Pourbaix version ] 2 [] 1 [ln 0 0.059 + + = + n M Mn n e e at 25°C (standard) Now pH is defined as pH = -log(H+) ∴ for the hydrogen half ...
how to read a pourbaix diagram vertical lines separate species that are in acid-base equilibrium. non vertical lines separate species related by redox equilibria. horizontal lines separate species in redox equilibria not involving hydrogen or hydroxide ions. diagonal boundaries separate species in redox equilibria in which hydroxide or …
Let me write that down. And there are many forms of phase diagrams. This is the most common form that you might see in your chemistry class or on some standardized test, but what it captures is the different states of matter and when they transition according to temperature and pressure. This is the phase diagram for water.
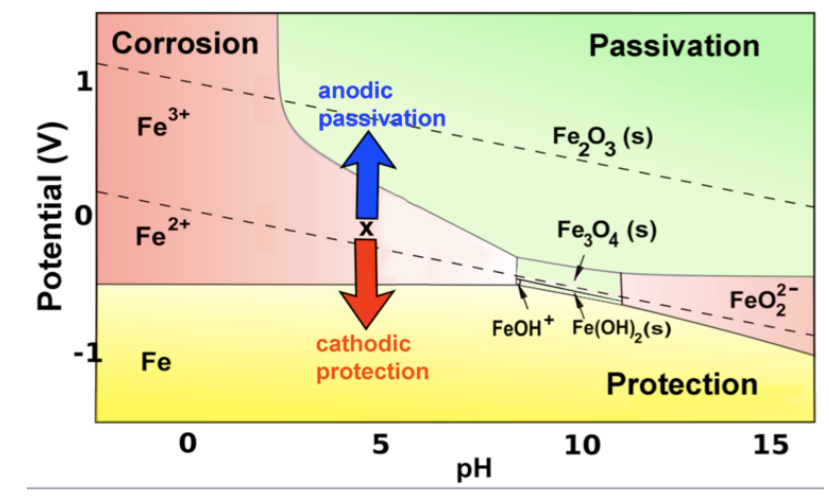
The 'Pourbaix' diagram was published in the 1950's and was hailed as validation of the -0.850v criterion in relation to a copper/copper sulfate electrode. Fig. 2. The Pourbaix diagram of Iron in water. Problems
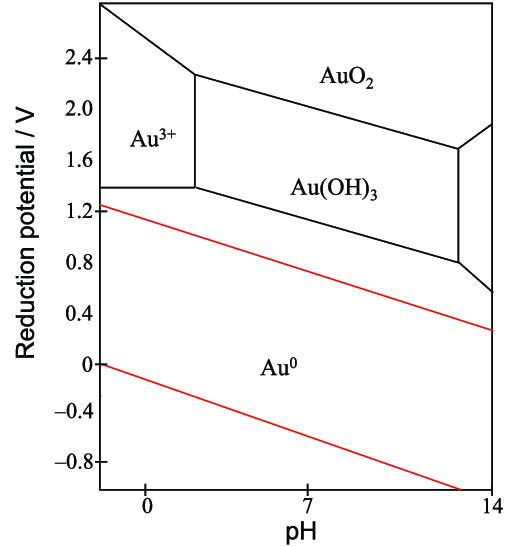
Uses of Pourbaix Diagrams: Any point on the diagram will give the termodynamically most stable (theoretically the most abundant) form of the element for that E and pH. E=+0.8 V and pH = 14 predominant form is FeO42-. The diagram gives a visual representation of the oxidizing and reducing abilities of the major stable compounds of an element
Pourbaix diagrams are useful in predicting the sponta-neous direction of electrochemical reactions, identifying the corrosion products and predicting the changes in environment in terms of potential and pH that result in high or low corrosive attack [8]. The present work provides Pourbaix diagrams for chromium carbides that can be used
March 22nd, 2019 - 3 1 How to read Pourbaix diagrams Pourbaix diagrams 1 also known as a potential pH diagrams show the possible stable equilibrium phases of a specific aqueous electrochemical system at a set temperature mostly at 298K Predominant ion boundaries are represented by lines As such a Pourbaix diagram can be read much like a ...
How to read pourbaix diagram. Read a phase diagram and explain its importance Identify phase equilibrium lines, triple points and critical points on a phase diagram Describe at what point a substance is a supercritical fluid 4.6: Pourbaix Diagram s Last updated; Save as PDF Page ID 183315; No headers.
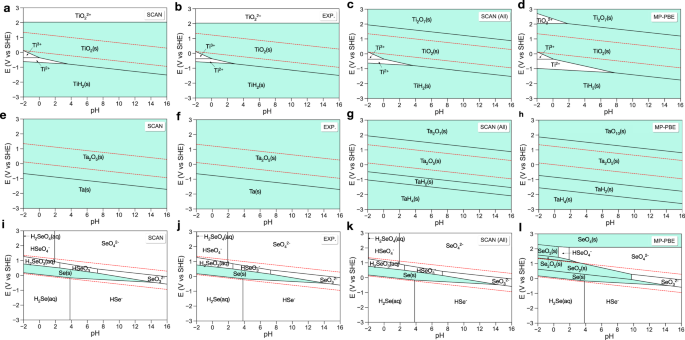
Predicting Aqueous Stability Of Solid With Computed Pourbaix Diagram Using Scan Functional Npj Computational Materials

Pourbaix Diagrams Of Alkaline Earth Metal Elements By Combination Of First Principles Calculations And Thermochemical Data Iopscience


















Comments
Post a Comment