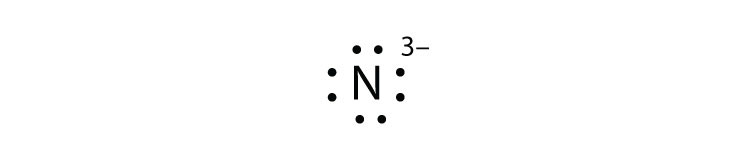
43 lewis dot diagram for zinc
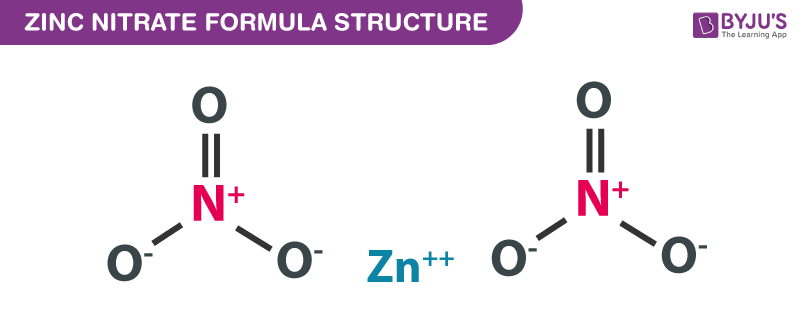
A step-by-step explanation of how to draw the Zn(NO3)2 Lewis Dot Structure.For Zn(NO3)2 we have an ionic compound and we need to take that into account when ... Zinc is #30. It has an electron configuration of [Ar] 4s2, 3d10. The 4s2 electrons are the bonding electrons. The dot structrue 2 dots. Zn:
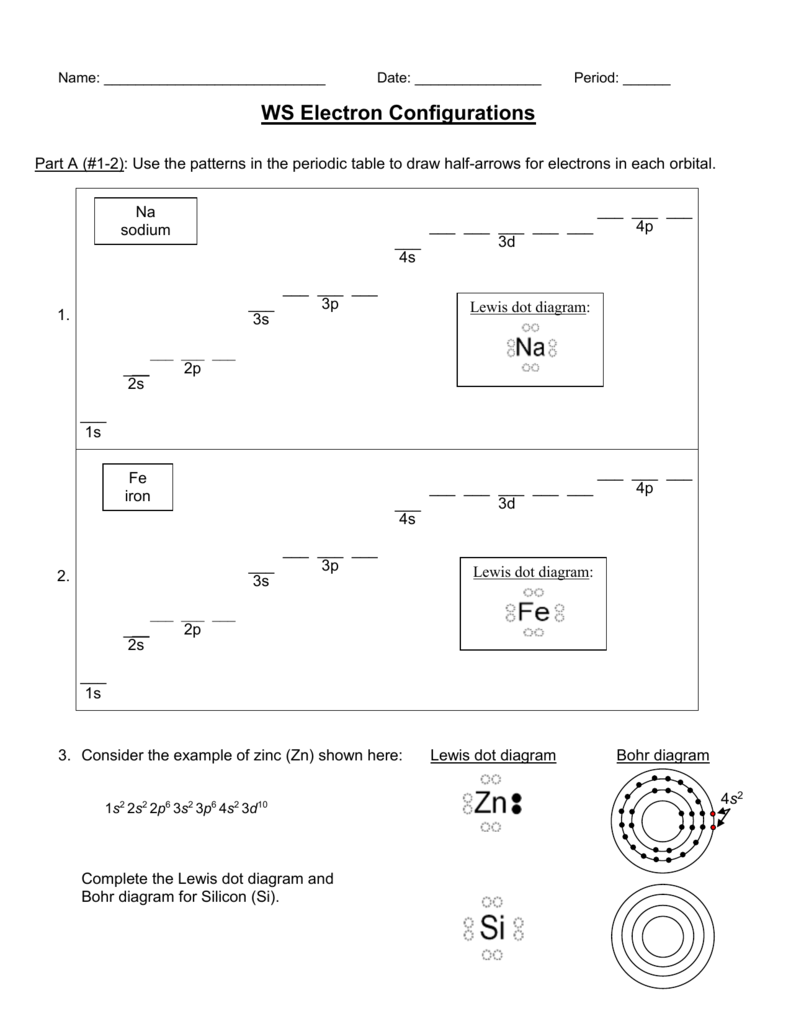
Consider the example of zinc (Zn) shown here: Lewis dot diagram Bohr diagram. 1s2 2s2 2p6 3s2 3p6 4s2 3d10. Complete the Lewis dot diagram and. Bohr diagram for Silicon (Si). Part B: Use the patterns in the periodic table to electron configurations for the following atoms. Symbol Group # Total # e– # valence e– (highest level) Electron ...

Lewis dot diagram for zinc
A step-by-step explanation of how to draw the Arsenic (As) Lewis Dot Structure.For the ArsenicLewis structure use the periodic table to find the total number... In zinc, these are the two 4s electrons, so in a Lewis structure zinc typically looks like Zn: 2. level 1. TangerineX. BS Comp Sci. 8 years ago. you typically don't make lewis dot structures of a singular atom. If you are looking for the Zn Chrystal, a lattice structure would be a better description of what the material looks like. Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral.
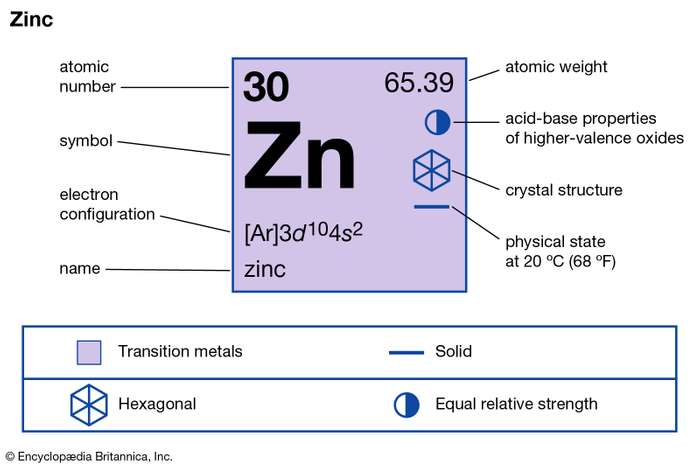
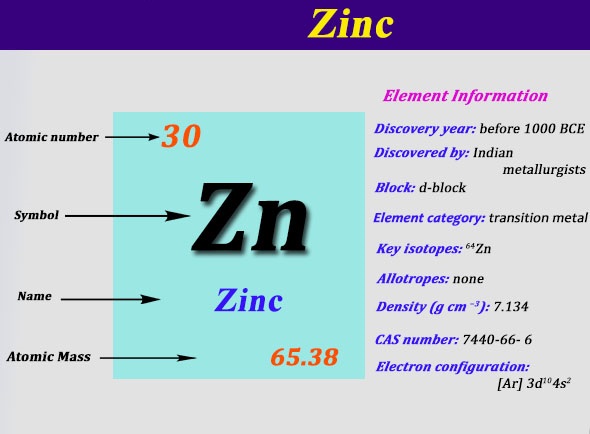
Lewis dot diagram for zinc. A step-by-step explanation of how to draw the Zn Lewis Dot Structure.For the Zn structure use the periodic table to find the total number of valence electron... The electron dot diagram for zinc is "Zn:" > Zinc (element number 30) is in the 4th Period of the Periodic Table. From left to right, you count two "4s" electrons and ten "3d" electrons. The "3d" shell is a filled inner shell, so only the "4s" electrons are valence electrons. Thus, the electron dot structure for zinc is "Zn:" A step-by-step explanation of how to draw the ZnBr2 Lewis Dot Structure.For ZnBr2 we have an ionic compound and we need to take that into account when we dra... Diagrams - Zinc. Bohr Diagram- Valence Electrons: 2. . Electron Configuration. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10. Noble Gas Configuration. Lewis Dot Diagram.
Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. In zinc, these are the two 4s electrons, so in a Lewis structure zinc typically looks like Zn: 2. level 1. TangerineX. BS Comp Sci. 8 years ago. you typically don't make lewis dot structures of a singular atom. If you are looking for the Zn Chrystal, a lattice structure would be a better description of what the material looks like. A step-by-step explanation of how to draw the Arsenic (As) Lewis Dot Structure.For the ArsenicLewis structure use the periodic table to find the total number...
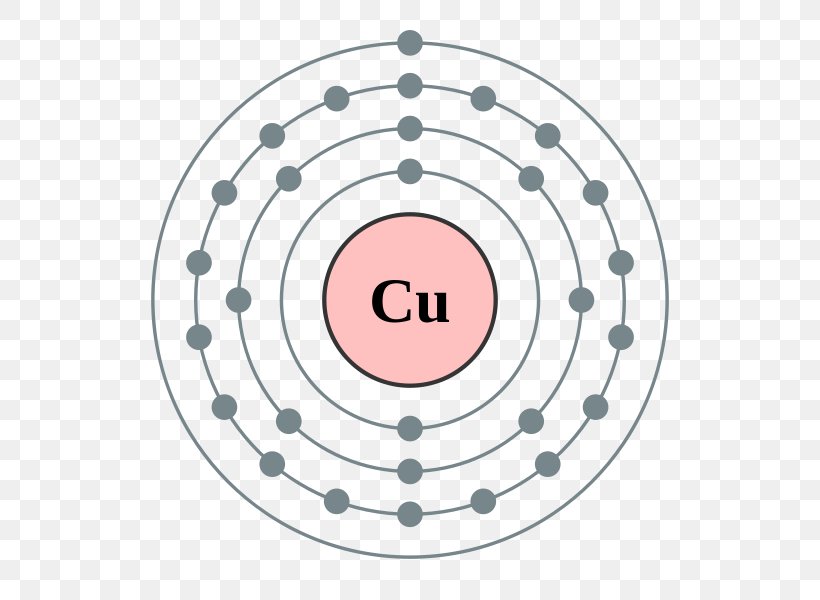
Electron Configuration Electron Shell Bohr Model Zinc Atom Png 600x600px Electron Configuration Atom Bohr Model Chemical

Chapters 15 6 Ionic Bonding 15 1 Objectives Use The Periodic Table To Infer The Number Of Valence Electrons In An Atom And Draw Its Electron Dot Lewis Ppt Download

Write The Electron Configuration For The Following Ci Zn Ca 2 F A Write One Type Of Chemical Bonds And One Type Of Chemical Reactions Example For Everyone B Write Lewis Structure For
















Comments
Post a Comment