43 pourbaix diagram copper
Pourbaix diagrams for the copper-chlorine system in the temperature interval 5 −100 oC have been revised. Predominance diagrams for dissolved copper containing species have also been calculated. Two different total concentrations of each dissolved element, 10 –4 and 10 –6 molal for copper and 0.2 and 1.5 molal for chlorine have been used in the Mar 10, 2018 · on Copper Pourbaix Diagram. The effects of pH on the form in which an element in a given oxidation state exists in natural waters can be summarized with predominance diagrams such as. Potential-pH diagrams are also called Pourbaix diagrams after the name of their Thus, Pourbaix diagrams introduce the concept of the following three states of . ABSTRACT Pourbaix diagrams (electrode potential-pH diagrams) for Cu-Br?-.
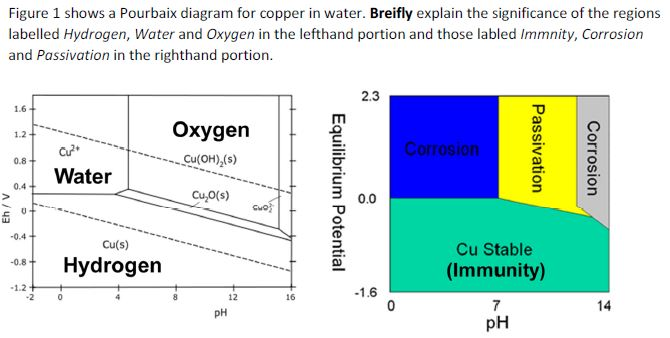
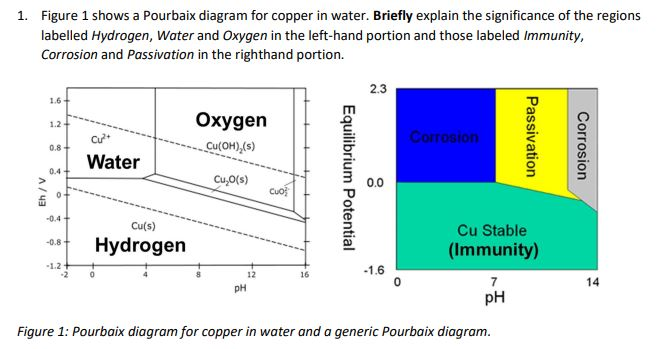
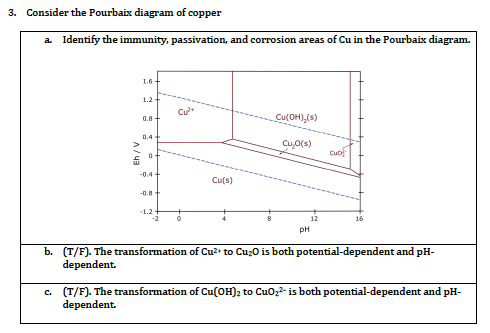
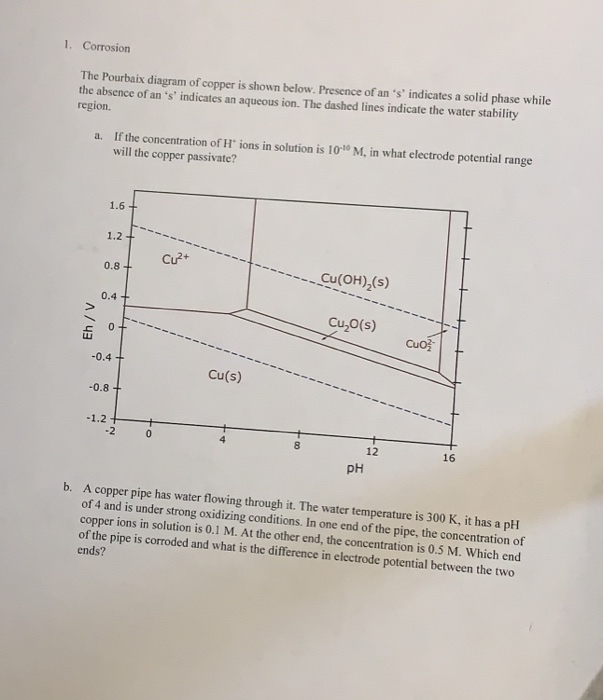
The local alkalization that occurs in the interface electrode solution can provoke the precipitation of CuO and Cu 2 O, as shown in the Pourbaix diagram for copper in an aqueous system (Fig. 1).
Pourbaix diagram copper
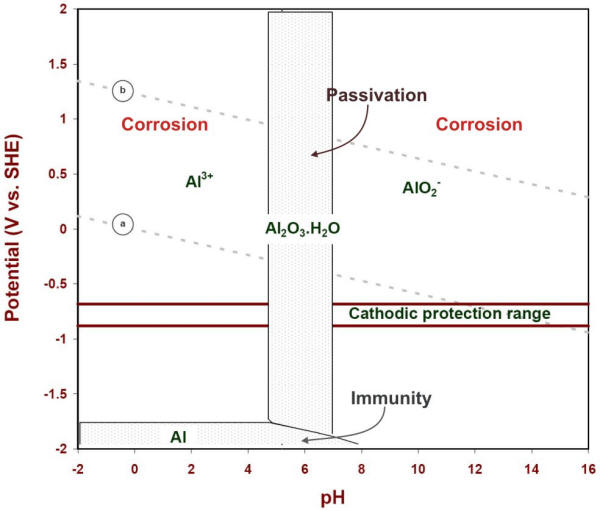
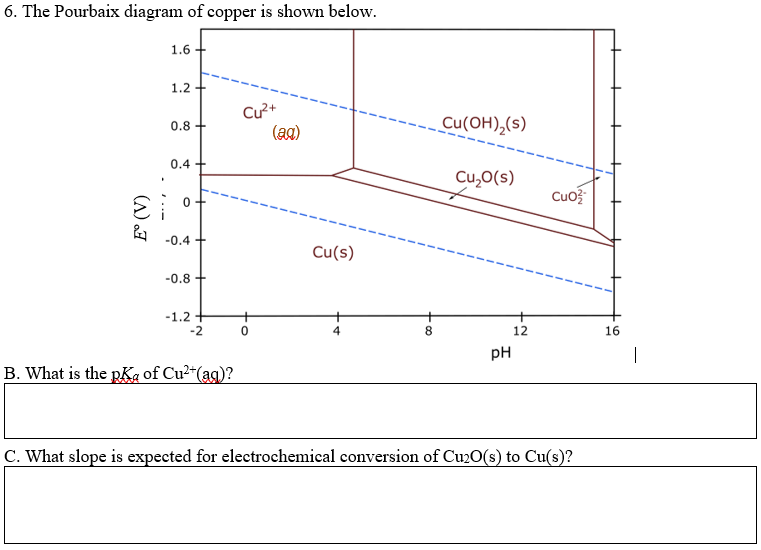
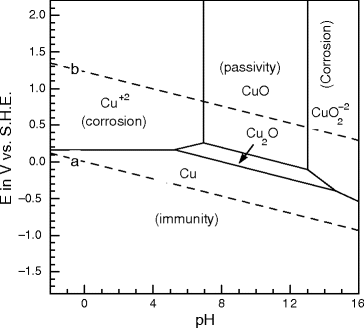
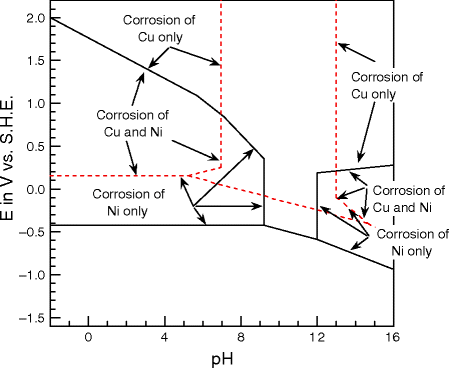
Pourbaix diagrams have several uses, including in corrosion studies. A Pourbaix diagram is also known as a potential/pH diagram, equilibrium diagram, EH-pH diagram, a pE/pH diagram on an E-pH diagram. The Pourbaix diagrams (potential-pH diagrams) for nickel at 25– °C have been revised. Extrapolation of thermodynamic data to elevated temperatures have been performed with the revised model of Helgeson-Kirkham-Flowers, which also allows uncharged .Pourbaix Diagrams for Copper in Aqueous ... In this example we derive the Pourbaix diagram for copper, accounting for the fact that both Cu(II) and Cu(I) change their composition at higher pH. approaching 8.5 and greater. The Pourbaix diagram in Fig. 3 [11] indicates stable forms of copper in aqueous solution as a function of pH and metal potential. For pH values below 7.0, the copper ion is stable in solution. Formation of the ion occurs from the oxide state when pH is just below 7.0. Replacement of the oxygen component
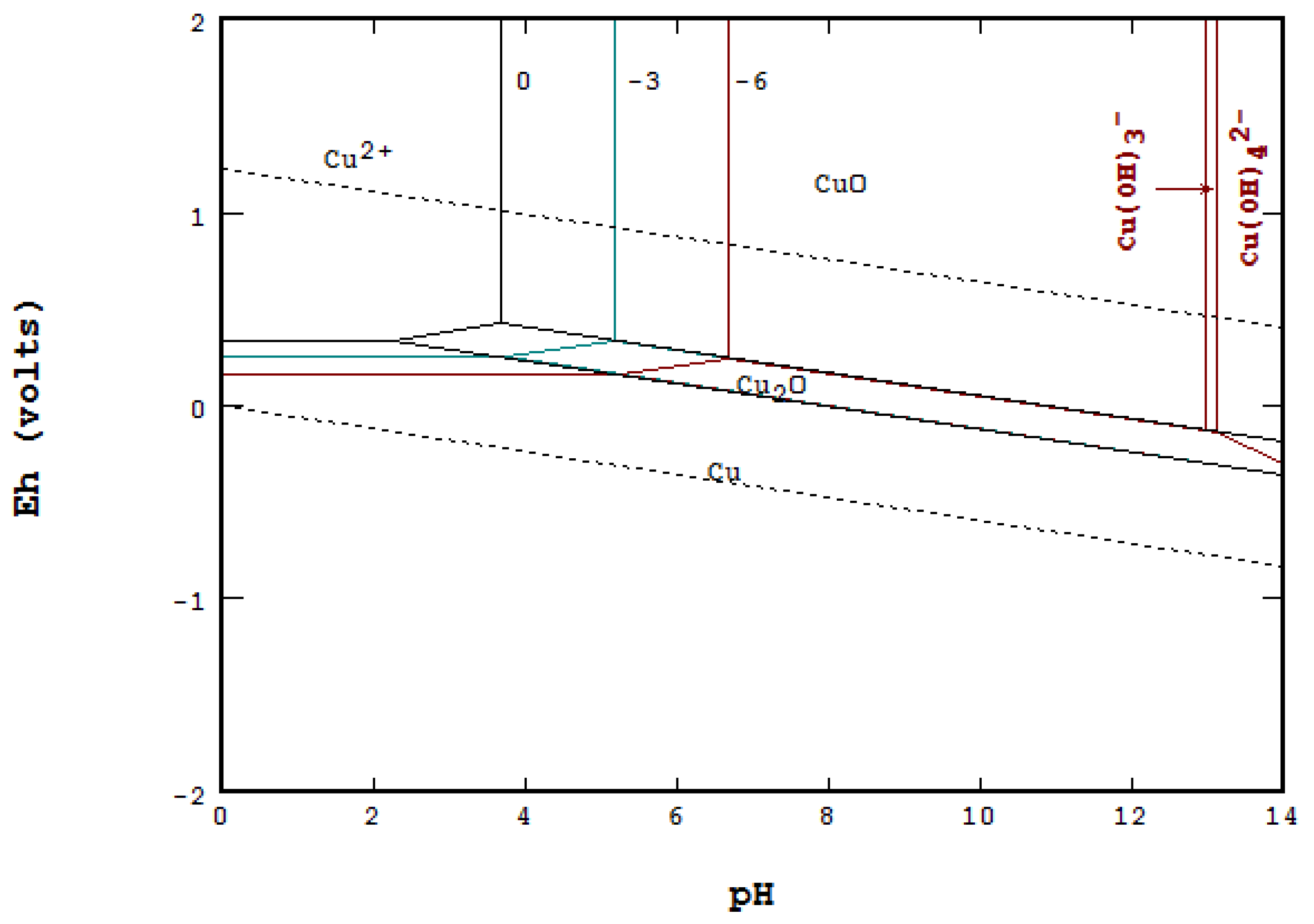
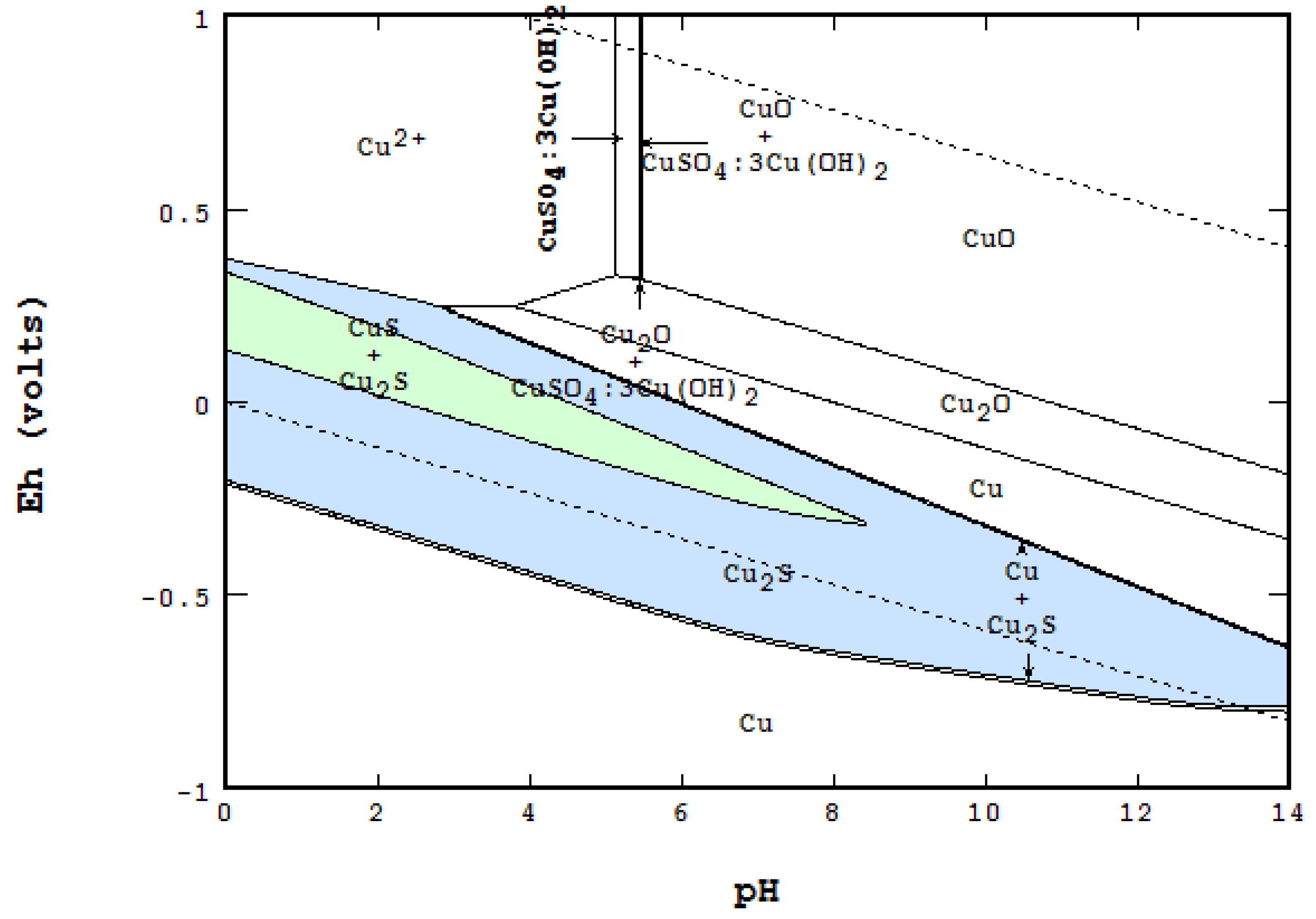
Pourbaix diagram copper. Pourbaix diagram for the copper-water-chloride system at 25°C for a chloride activity of 1 mol/kg (King and Kolar 2000). The shaded box shows the range of corrosion potentials in O 2-containing ... In general, copper is etched under acidic conditions because it is readily ionized to Cu 2+ , as can be seen in the Pourbaix diagram for 15 Cu-H O systems shown in Fig. 1. The Pourbaix diagram is here. My Answer: For a solution saturated with hydrogen and in the alkaline region of the Pourbaix diagram, copper is a solid in a region of stability. Therefore, copper pipe is a good idea as there will be no oxidation of the copper pipe to CuO2 and copper ions will not be entering the KOH solution. Jan 17, 2019 · Pourbaix diagram for copper in uncomplexed media (anions not other than OH- considered). Ion concentration m (mol/kg water).Pourbaix diagrams for (A) copper in water, (B) copper in water with a total activity of BTAH of 10 −4, and (C) copper in water with a total activity of BTAH of 10 −2. The cross-hatched region indicates Cu 2 O.
O system showing its pourbaix diagram at 25 oC (House, 1987). Native copper and sulphides requires an oxidising agent and acid to be leached while minerals such as CuO can be leached by lowering the pH. (b) Cu-Fe-S-O-H 2 O system pourbaix diagram at 25 oC (Peters, 1976). The requirement of both an oxidising agent and acidic environment is approaching 8.5 and greater. The Pourbaix diagram in Fig. 3 [11] indicates stable forms of copper in aqueous solution as a function of pH and metal potential. For pH values below 7.0, the copper ion is stable in solution. Formation of the ion occurs from the oxide state when pH is just below 7.0. Replacement of the oxygen component In this example we derive the Pourbaix diagram for copper, accounting for the fact that both Cu(II) and Cu(I) change their composition at higher pH. Pourbaix diagrams have several uses, including in corrosion studies. A Pourbaix diagram is also known as a potential/pH diagram, equilibrium diagram, EH-pH diagram, a pE/pH diagram on an E-pH diagram. The Pourbaix diagrams (potential-pH diagrams) for nickel at 25– °C have been revised. Extrapolation of thermodynamic data to elevated temperatures have been performed with the revised model of Helgeson-Kirkham-Flowers, which also allows uncharged .Pourbaix Diagrams for Copper in Aqueous ...
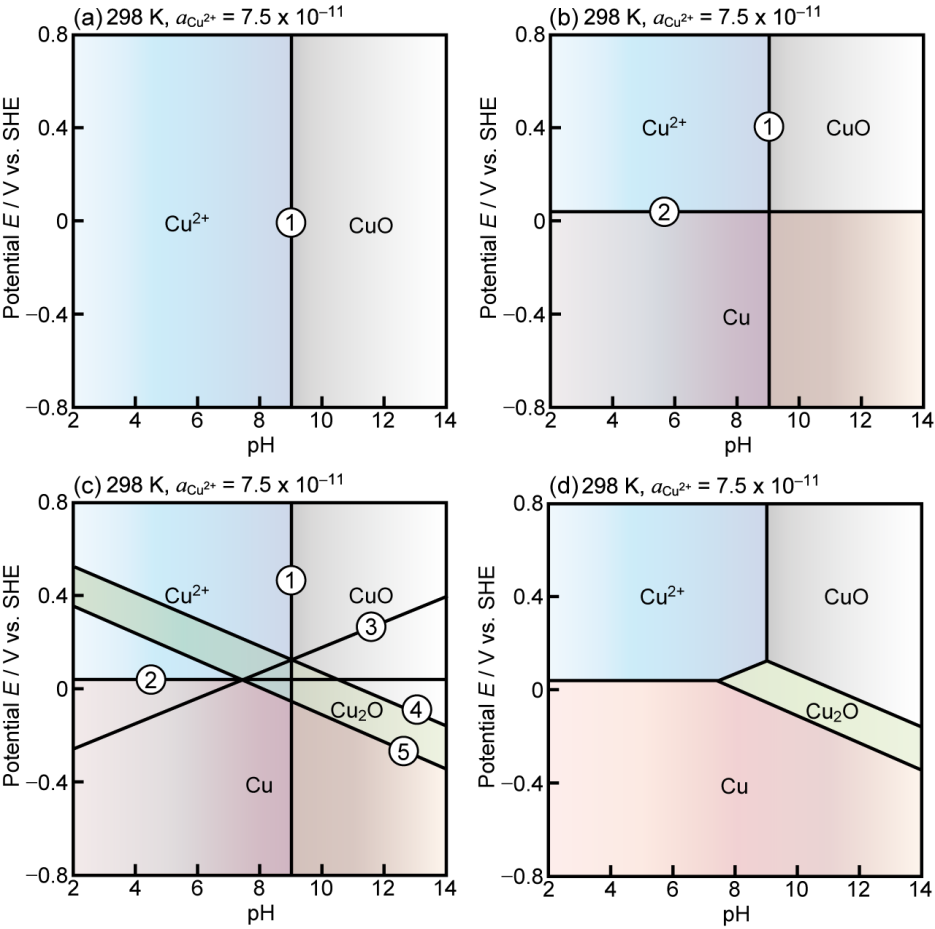
Potential Ph Diagrams Considering Complex Oxide Solution Phases For Understanding Aqueous Corrosion Of Multi Principal Element Alloys Npj Materials Degradation

Potential Ph Diagrams For Oxidation State Control Of Nanoparticles Synthesized Via Chemical Reduction Intechopen



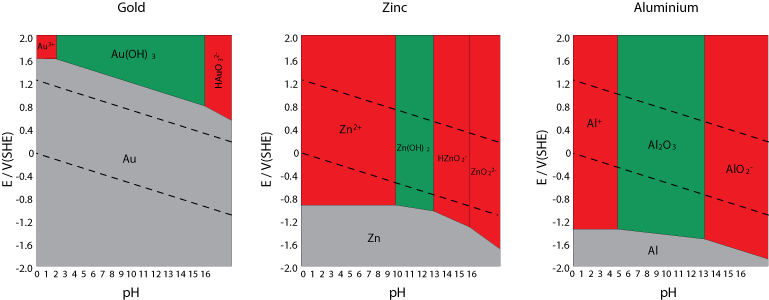












Comments
Post a Comment