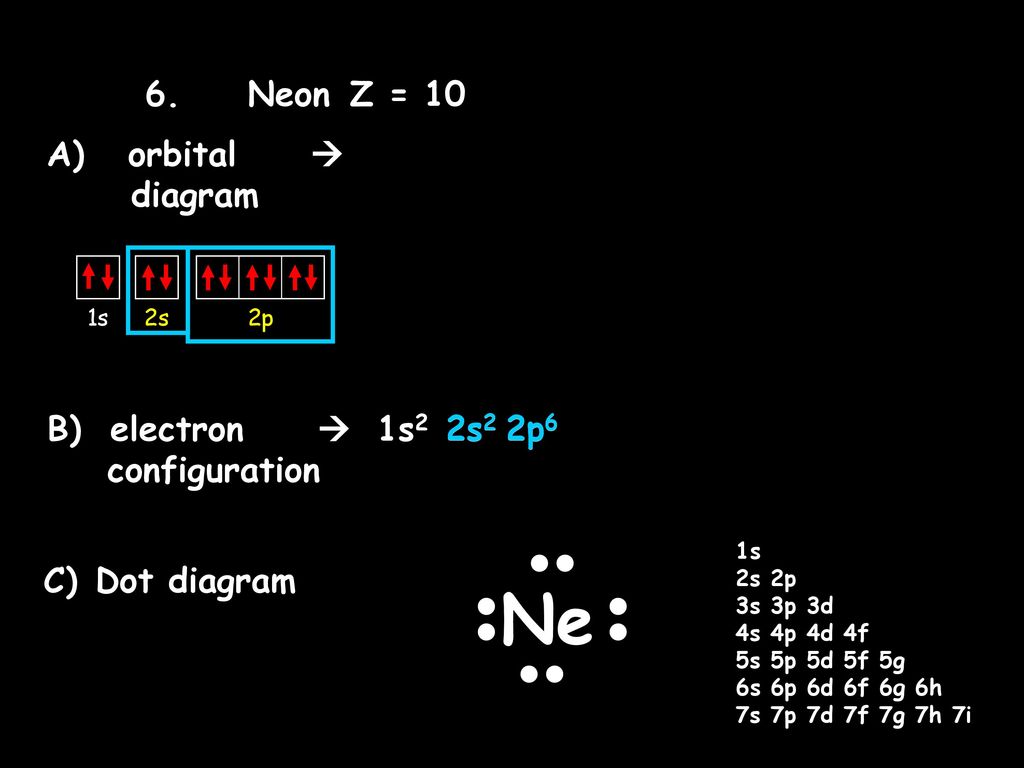
39 orbital diagram for ne
Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... Answered: 1. Does the molecule Ne, exist? A. Draw… | bartleby 1. Does the molecule Ne, exist? A. Draw a molecular orbital energy diagram for Ne2. B. Write the molecular orbital occupation for Ne,. C. Calculate the bond order for Ne, D. Using MO Theory, do you expect Ne, to be a stable molecule? Explain why or why not. E. Using MO Theory, is Ne, diamagnetic or paramagnetic?
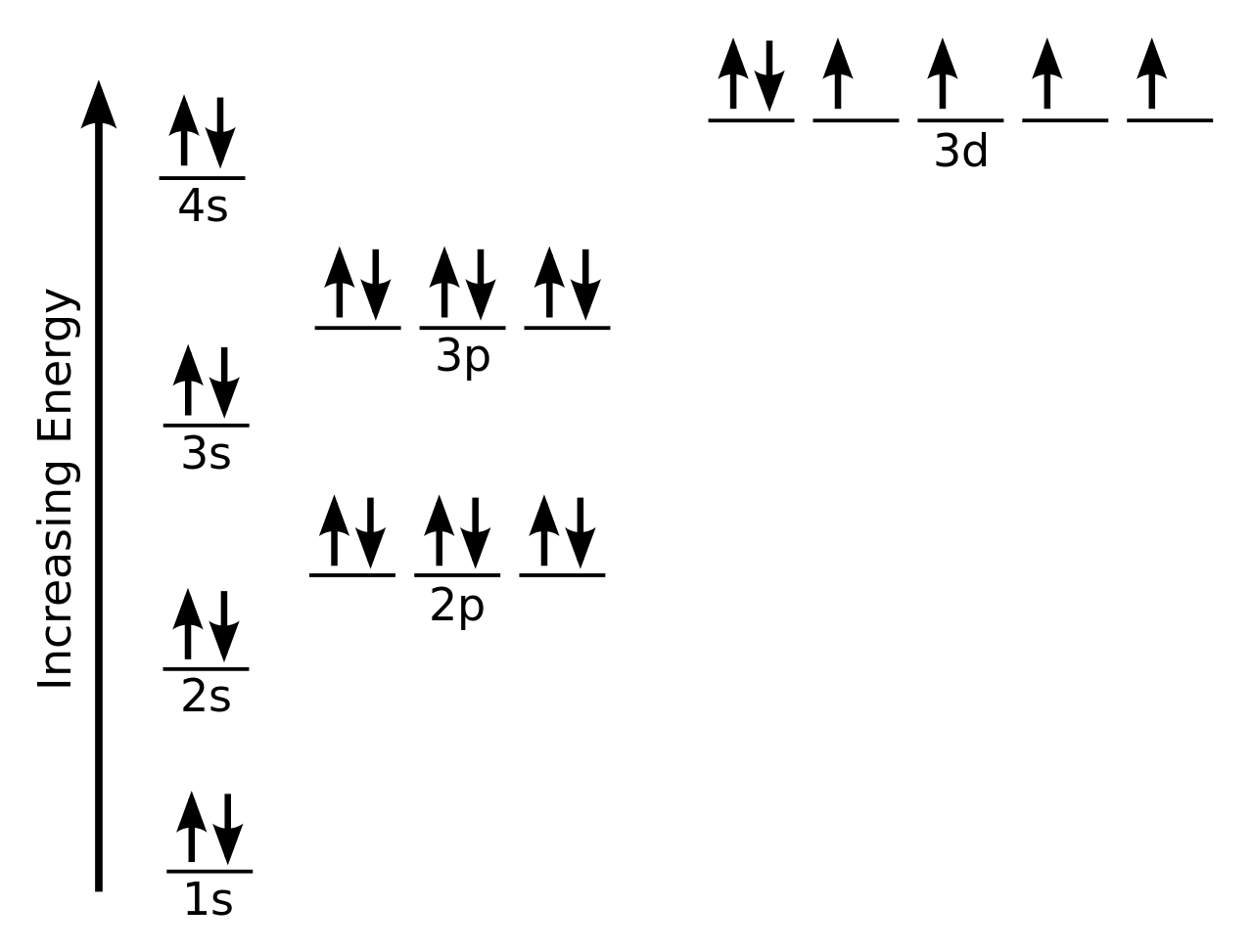
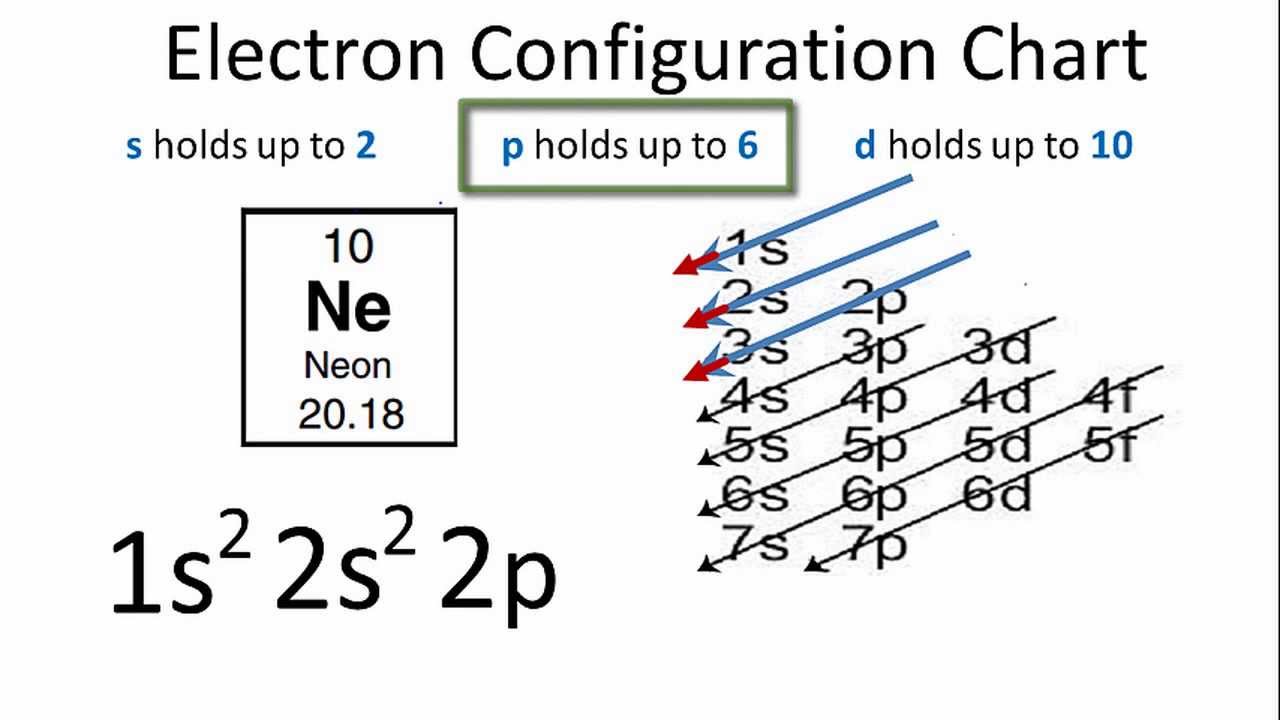
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom

Orbital diagram for ne
Molecular Orbital Diagram For Ne2 According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or. Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. © Prof Adam J Bridgeman | close window. SOLVED:Write full orbital diagram for Ne_ Use the buttons ... Write full orbital diagram for Ne_ Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons 1s 2s 2p] 38 3p 3d 4p 4d Gs (Sp Sd 5f 0s (6p} 6d Vp 7d 2p ##HF Get the answer to your homework problem. Orbital Diagrams Flashcards | Quizlet The order in which orbitals are listed on an orbital diagram follows: The Aufbau principal Hund's rule states that the electron configuration with the lowest-energy will have the maximum possible number of unpaired electrons.
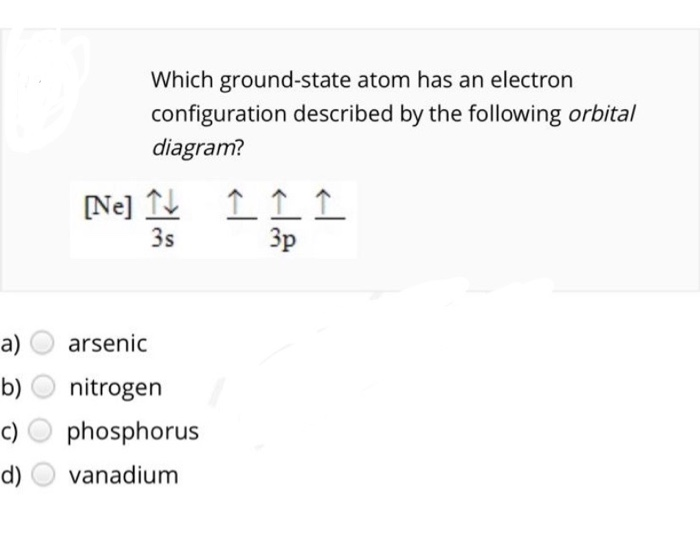
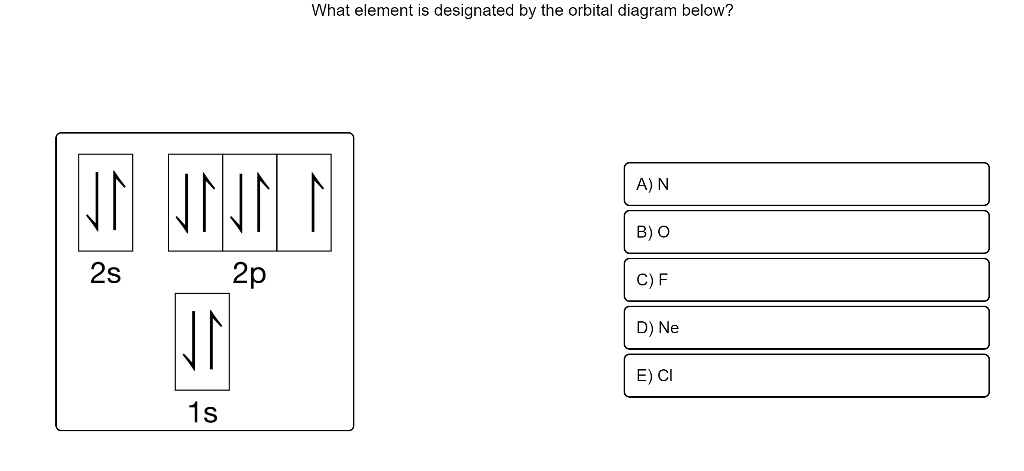
Orbital diagram for ne. Solved Which ground-state atom has an electron ... - Chegg Question: Which ground-state atom has an electron configuration described by the following orbital diagram? [Ne] Tv 3s 소 소 소 Зр a) arsenic b) c) d) nitrogen phosphorus vanadium . This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text PDF ELECTRONIC STRUCTURE OF ATOMS - Los Angeles Mission College Z Orbital Diagram Electron Configuration 10 Ne 1s 2s 2p 1s 2 2s 2 2p 6 or [He] 2s 2 2p 6 2e's 8 e's NOTE: The 1 st and the 2 nd energy levels are complete (filled to capacity) 11 Na 1s 2s 2p 3s 1s 2 2s 2 2p 6 3s 1 or [Ne] 3s Neon(Ne) electron configuration and orbital diagram Neon (Ne) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. Molecular Orbital Diagram Ne2 - schematron.org For Ne 2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 schematron.org each MO an appropriate label. Determine the electron configuration and bond order for each, and rank the three species in order of increasing bond order% (1).
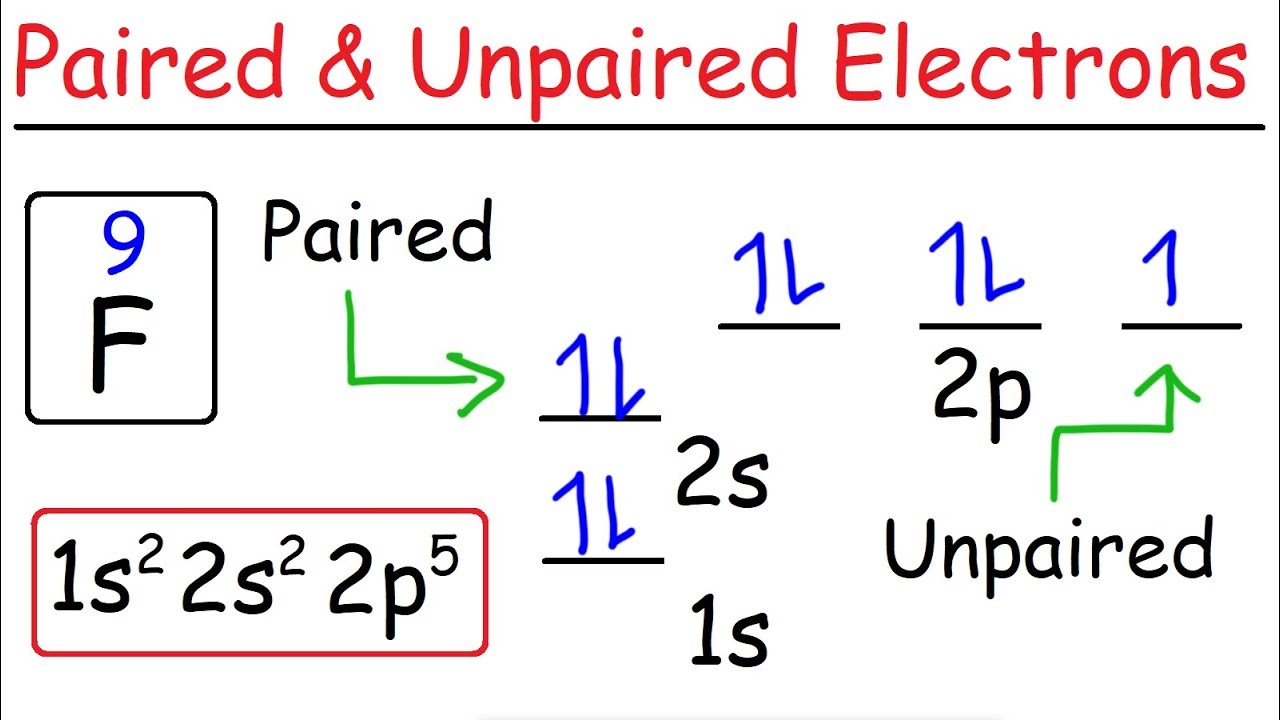
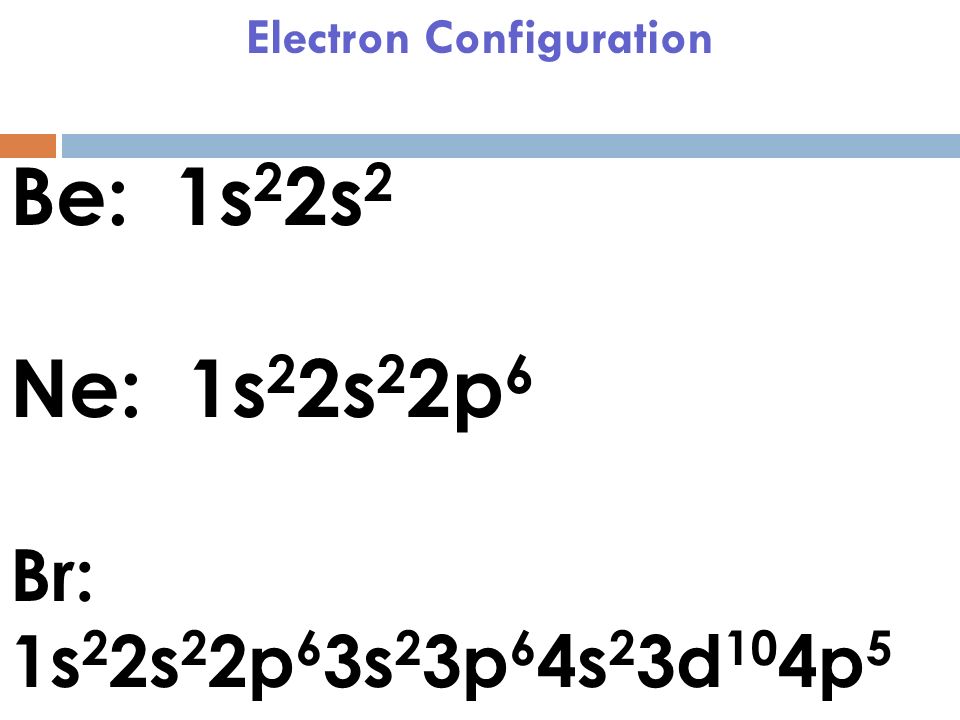
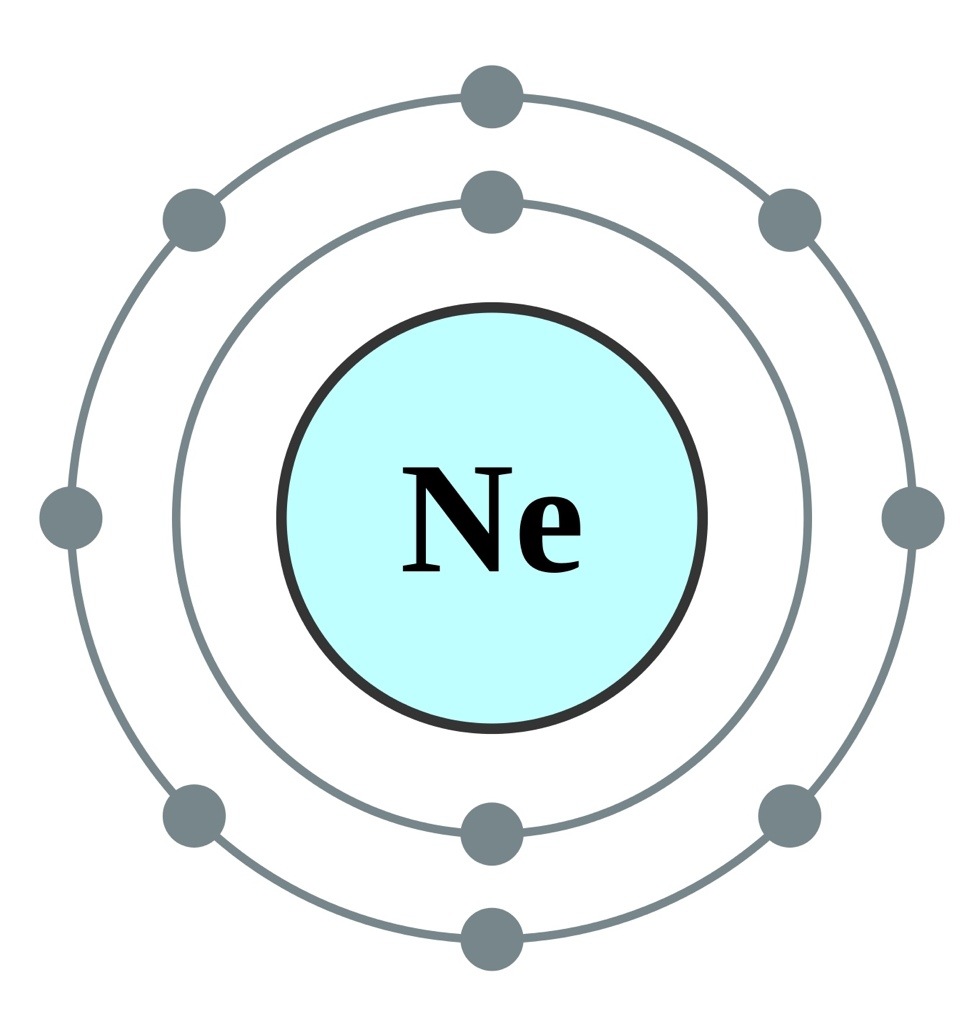
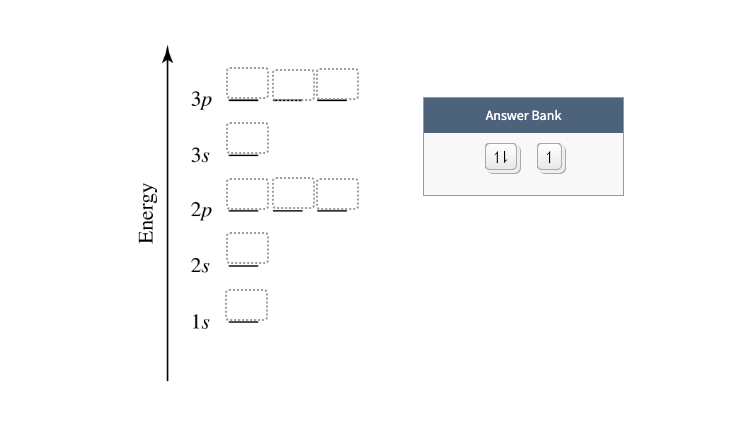
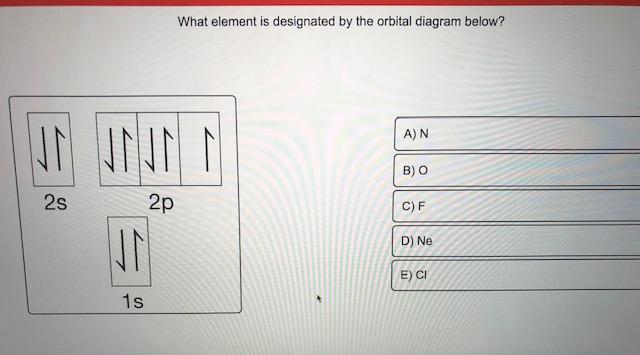
What is the orbital diagram for Helium? - AskingLot.com Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Electron Configuration for Neon (Ne) - UMD Neon is the tenth element with a total of 10 electrons. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. The remaining six electrons will go in the 2p orbital. Neon (Ne) Orbital diagram, Electron configuration, and ... The orbital diagram for Neon is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The Neon orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the remaining six electrons in 2p orbital. Orbital diagram for a ground-state electron configuration of Neon atom is shown below- Properties and Uses of Neon Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ...
Molecular Orbital Diagram For Ne2 - Wiring Diagram Pictures Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable. PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine Orbital Diagram For Calcium (Ca) | Calcium Electron ... The 6 electrons will go to the 2p orbital, and the next 2 electrons will place with 3s orbital, and now we have only 8 electrons in which 6 electrons will go with 3p orbital, and the last 2 electrons will be with 4s orbital. So, we have Calcium Electron Configuration is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s². So, the configuration helps to know the ... Orbital Diagrams Chemistry Tutorial - AUS-e-TUTE condensed electron configuration: [Ne] 3s 2. orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z boxes, with 2 electrons placed in the 3s box Apply the Pauli Exclusion Principle so that for paired electrons, one electron has "up spin" and the other has "down spin" ↑↓ ↑↓ ↑↓ ↑↓
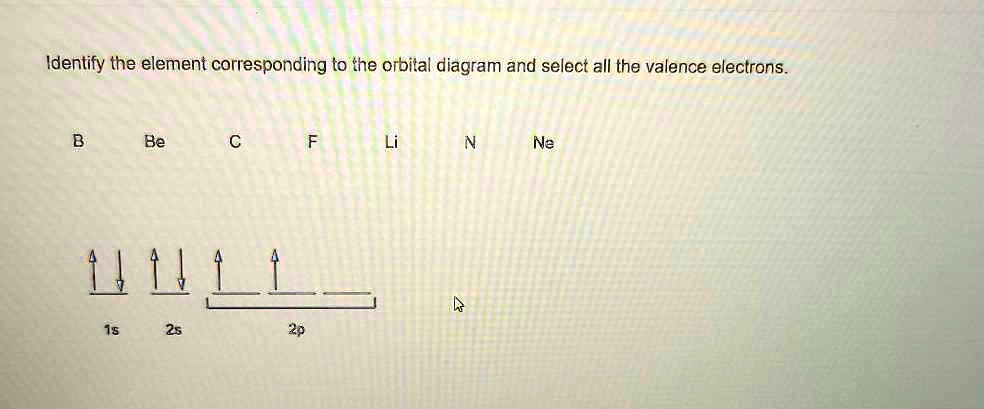
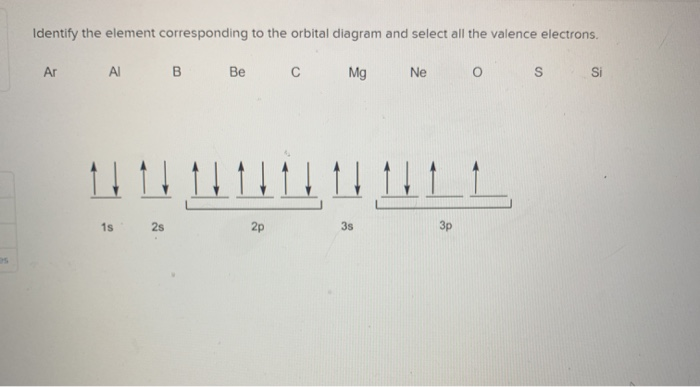
Solved Part A Write orbital diagrams for the valence ... - Partc Write orbital diagrams for the valence electrons of I Drag the appropriate; Question: Part A Write orbital diagrams for the valence electrons of Ne. Drag the appropriate labels to their respective targets. Not all labels will be used. 03 QORIDODOO G2 Part B Indicate the number of unpaired electrons in Ne. Express your answer as an Integer.
Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration February 15, 2021 by Sneha Leave a Comment Nitrogen Electron Configuration : When we talk about school subjects, then one of the major subjects which are very important for knowledge perspective is science .
Sodium(Na) electron configuration and orbital diagram Sodium (Na) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
How to Write the Orbital Diagram for Neon (Ne) - YouTube To write the orbital diagram for the Neon atom (Ne) first we need to write the electron configuration for just Ne. To do that we need to find the number of ...
Memahami Konfigurasi Elektron dan Diagram Orbital Lebih ... Diagram Orbital. Nah sekarang kita akan menggambarkan konfigurasi elektron memakai diagram orbital, teman. Sebenarnya gambarnya cukup mudah kok. Suatu subkulit punya sejumlah orbital. Orbital itu digambarkan sebagai persegi dan berisi garis setengah panah yang mewakili elektron. Subkulit s punya 1 orbital, p punya 3 orbital, d punya 5 orbital ...
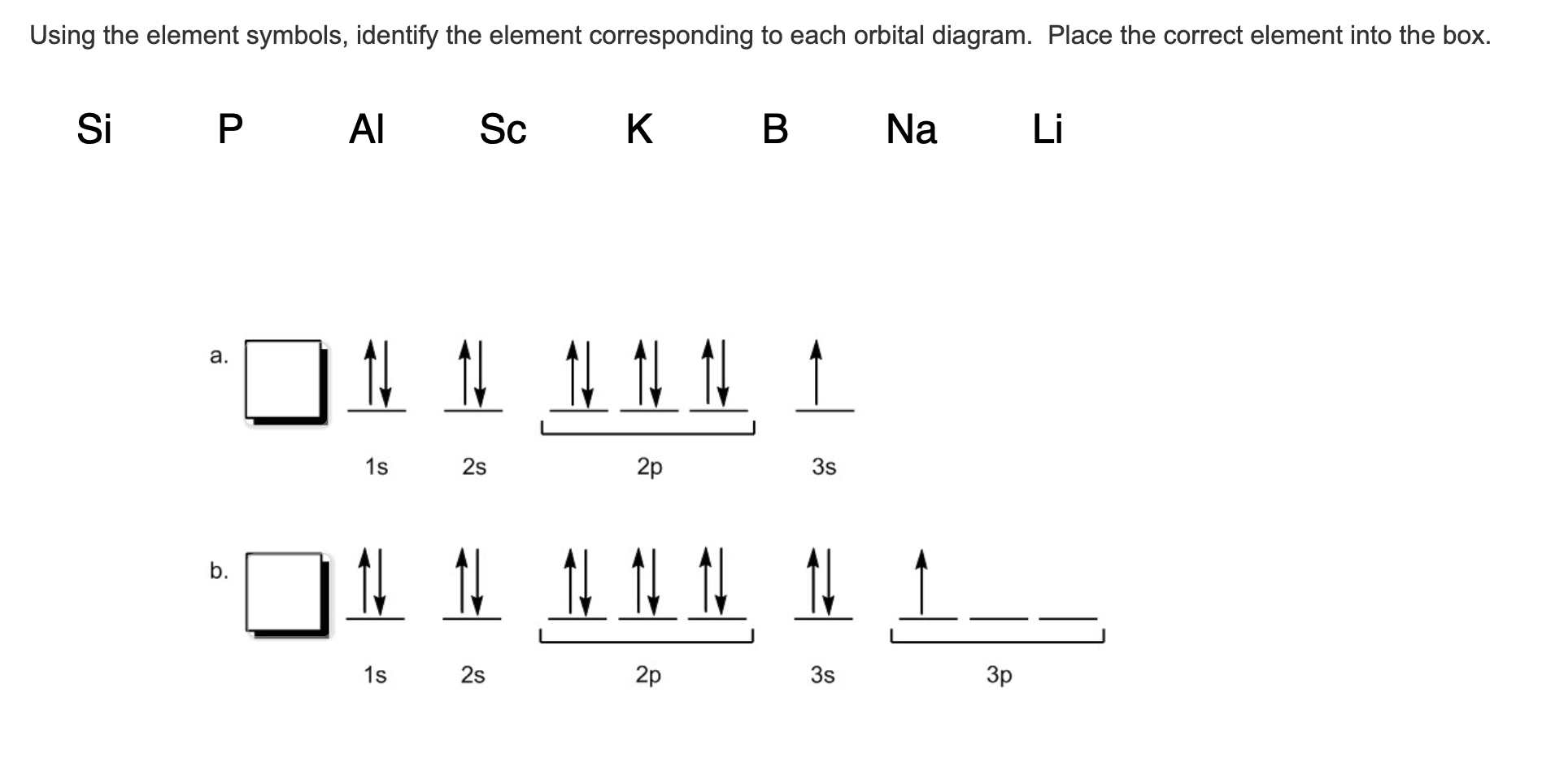
Aluminum Orbital diagram, Electron configuration, and ... The orbitals are 1s, 2s, 2p, 3s, and 3p. The Aluminum orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, the two electrons in 3s orbital, and the remaining one electron in 3p orbital. Orbital diagram for a ground-state electron configuration of an Aluminum atom is shown below-.
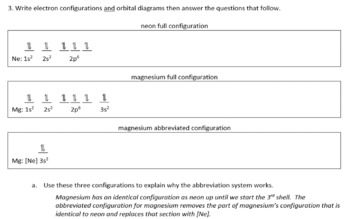
PDF Electron Configurations and Orbital Diagrams key 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? The probability of finding an electron at the nucleus is 0 (you will never find an electron in the nucleus). 3. 2The electron configuration for phosphorus, written in core notation, is [Ne] 3s 3p 3. What two things does Hund's rule tell us
Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows. Energy levels: 2, 8, 6 Orbitals: 1s2 2s2 2p6 3s2 3p4 If you need to fill in the little boxes, here's one for you. Each arrow represents one electron.
What is the orbital diagram for neon? - Answers An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in...
Write the full orbital diagram for Ne. | Study.com Write the full orbital diagram for Ne. Orbital Diagram: One can easily show the electronic arrangement in different orbitals of an element, using the basic concept of orbital diagram.
Molecular orbital diagram - WikiMili, The Best Wikipedia ... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. [1] [2] [3] A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the ...
Orbital Diagrams Flashcards | Quizlet The order in which orbitals are listed on an orbital diagram follows: The Aufbau principal Hund's rule states that the electron configuration with the lowest-energy will have the maximum possible number of unpaired electrons.
SOLVED:Write full orbital diagram for Ne_ Use the buttons ... Write full orbital diagram for Ne_ Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons 1s 2s 2p] 38 3p 3d 4p 4d Gs (Sp Sd 5f 0s (6p} 6d Vp 7d 2p ##HF Get the answer to your homework problem.
Molecular Orbital Diagram For Ne2 According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or. Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. © Prof Adam J Bridgeman | close window.
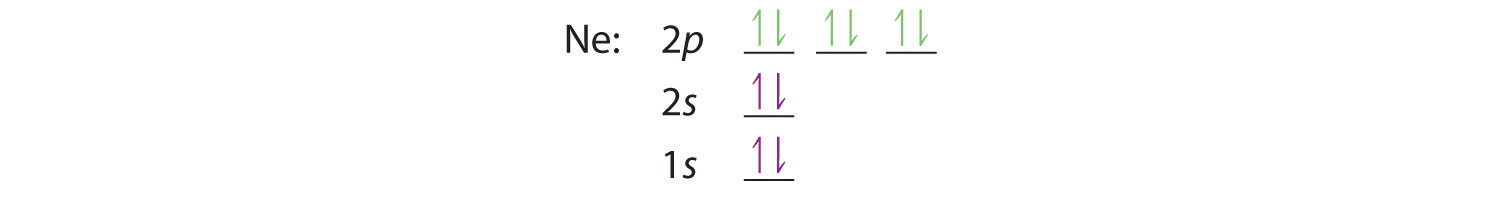


![Example for Na [Ne] 3s1 Na = 1s2 2s2 2p6 3s1 electron ...](https://slideplayer.com/13828450/85/images/slide_1.jpg)




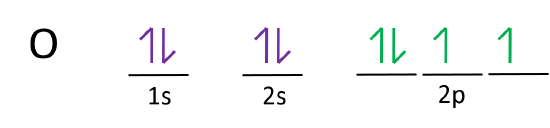



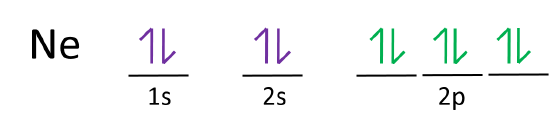






Comments
Post a Comment